Sodium butyrate-induced death-associated protein kinase expression promote Raji cell morphological change and apoptosis by reducing FAK protein levels1
Introduction
Sodium butyrate is a short chain, fatty acid sodium salt that was produced by the carbohydrate fermentation of bacteria. The concentration of sodium butyrate can attain 5 mmol/L in the human colon. Evidence has shown that sodium butyrate was the major fuel for colonic epithelial cells, and it can influence cell proliferation by the release of growth factors or gastrointestinal peptides, such as gastrin, or by the modulation of mucosal blood flow[1]. It was suggested that sodium butyrate had a weak toxicity to normal cells.
Sodium butyrate causes the histone acetylation by inhibiting the activation of histone deacetylase. Sodium butyrate is not only a deacetylase inhibitor, but also has the function of demethylation[2–4].
Sodium butyrate could induce the transformation of cell differentiation or apoptosis[2,3]. Heerdt et al demonstrated that sodium butyrate and its analogue could arrest MCF-7 cell growth. They prompted cell apoptosis by the mitochondrial pathway[2]. Duan et al also demonstrated that sodium butyrate and trichostatin A (TSA) induced apoptosis and downregulated the transcription of Bcl-2[5].
Recently, Earel et al showed that sodium butyrate and trichostatin A (TSA) increased TNF-related apoptosis-inducing ligand receptor 2 gene transcription to accelerate the death-inducing signaling complex formation, caspase activation, and loss of mitochondrial membrane potential of tumor cells[6].
The above results showed that sodium butyrate was a novel chemotherapeutic drug with weak toxicity; however, the precise mechanism has not been elucidated.
Raji cells were derived from a Burkitt lymphoma. Normally, Raji cells are suspended and grow in medium. Death-associated protein kinase (DAPK), which is a Ca2+/CaM regulation protein kinase and executes program cell death in various signal transduce pathways, was silenced in the Raji cells because of the methylation of the DAPK gene promoter[7]. In our earlier studies, we reported that Raji cells expressed DAPK and displayed protrusions to adhere to matrices induced by sodium butyrate[8].
In the present study, we determined whether DAPK expression would prompt Raji cell apoptosis and whether adhesion-growth Raji cells would be more sensitive to anoikis than suspended-growth Raji cells, thus, we investigated its possible mechanism.
Materials and methods
Cell culture The Raji cell line, offered by Sun Yat-Sen University Cancer Center (Guangzhou, Guangdong, China) was cultured in the RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA) containing penicillin 100 µg/mL and streptomycin 100 µg/mL and supplemented with 10% calf blood serum (Sijiqing Laboratories, Hangzhou, China) at 37 °C in a humidified atmosphere with 5% CO2.
Effect of sodium butyrate on the morphology of Raji cells The Raji cells were cultured in 6-well plates (3.0×106 cells/well) and treated with 3 mmol/L sodium butyrate (Sigma, St Louis, MO, USA) at 37 °C in a humidified atmosphere with 5% CO2 for 2 d. Cell morphology was observed with a scanning electron microscope (JSM-T300, Shimadzu,Kyoto, Japan) at the Guangzhou Medical College Electron Microscope Center (Guangzhou, China).
Effects on apoptosis by preventing Raji cells adhesion Each well of the 24-well plates was covered with 1mL polyHEME (10 mg/mL; Sigma, USA) dissolved in ethyl alcohol. After the ethyl alcohol had been air-dried, each well of the 24-well plates was washed with 1×phosphate-buffered saline (PBS)[9].
The Raji cells were divided into 3 groups. The first group was cultured in polyHEME-coated, 4-well plates and named the polyHEME group; the second group was cultured in the normal 24-well plates and named the non-polyHEME group; the third group was the control group. The cell number of every well was 2.0×105 cells. The polyHEME and non-polyHEME groups were treated with 3 mmol/L sodium butyrate for 2, 4, 6, 8, and 10 d, separately. The control group was cultured in the normal medium. The cells in the control group were supplied with 0.5 mL medium every 4 d. Simultaneity, the sodium butyrate-treatment group cells were supplied with 0.5 mL medium containing 3 mmol/L sodium butyrate.
Some cells samples were taken from each well to determine cell viability by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, USA) assay every 2 d[10]. Briefly, the number of viable cells in each well was estimated by adding 10 µL 0.5 mg/mL MTT solution. The cells were dissolved with 100 µL solution that contained 20% SDS and 50% dimethyl formamide after the cells had been incubated for 4 h at 37 oC. The optical densities were quantified at 570 nm (Bio-Rad model 540, Hercules, CA, USA). The results were estimated by absorbency. In each well, the remains of the cells were centrifuged and washed twice in 1× PBS and incubated in 1× PBS containing 100 µg/mL propidium iodide (PI) (Sigma, USA) and 200 µg/mL RNase after the cells were fixed with ice-cool 70% ethanol overnight. The cells were then analyzed at an excitation wavelength of 488 nm by flow cytometry (EPICS XL, Coulter, Fullerton, CA, USA). The percentage of degraded DNA was determined by the number of cells with subdiploid DNA divided by the total number of cells examined under each experimental condition[10].
A morphological study was performed as described previously[11]. After being treated with sodium butyrate, the cells were collected, washed with 1× PBS, and incubated in 1×PBS containing 10 µg/mL Hoechst33258 (Sigma, USA) for 10 min. Nuclear morphology was examined using fluorescence microscopy (Olympus DX71, Olympus Corporation, Tokyo, Japan).
DAPK and FAK expression analysis in Raji cells The cells were loaded with cell decomposition buffer (pH 8.0) that contained 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% NP40, 0.05% phenylmethanesulfonyl fluoride(PMSF), 2 µg/mL aprotinin (Sigma, USA), and 2 µg/mL leupeptin (Sigma, USA) after centrifugation and washed twice in 1×PBS. The cells were then treated 3 times for 10 s with ultrasonic processing on ice. The proteins were determined as described previously by Western blotting[12] using the DAPK monoclonal antibody (clone-55, Sigma, USA), rabbit antihuman FAK (C-20, Santa Cruz Biotechology, Santa Cruz, California USA), anti-actin (pan Ab-5, clone actn05, Lab Vision, Fremont, CA, USA), and Western blotting luminol reagent (Amersham Biosciences, Uppsala, Sweden).
Construction of expression plasmid for C-terminal peptides of DAPK The C-terminal coding peptides of DAPK were amplified from pcDNA3.1(+)-DAPK[13] with the PCR method and subcloned into the expression vector pcDNA3.1(+), forming pcDNA3.1(+)-DCTP[13].
The Raji cells were transfected with a pcDNA3.1(+)-DCTP using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s protocol. Stable cell lines were cultured in medium containing 1000 µg/mL G418 (Invitrogen, USA). Three weeks later, the cells were cultivated in the medium that contained 500µg/mL G418. The Raji cells transfected with pcDNA3.1(+)-DCTP were induced by sodium butyrate. The morphology of the cells was observed under a microscope (Olympus DX71) and cell apoptosis was detected by flow cytometry.
Data analysis Data were expressed as mean±SD. Statistical significance was evaluated using the Student’s t-test. P<0.05 was considered to be statistically significant.
Results
Effect of sodium butyrate on Raji cells After being treated with sodium butyrate, the suspended-growth Raji cells adhered to the bottom of the culture flask (Figure 1). The newborn fibrous pseudopods structures occurred on the cell.

Effect on cell growth by preventing Raji cell adhesion After being treated with sodium butyrate, the survival of the Raji cells was reduced in a time-dependent manner if cell adhesion was suppressed (Figure 2). In contrast, the cells treated with sodium butyrate continued to grow slowly if the cell adhesion was permitted. The control group cells proliferated so rapidly that they attained to plainness phase in 4 d.

Suppressing adherence increased the apoptotic Raji cell ratio The apoptotic cells induced by sodium butyrate increased in a time-dependent manner. Significant DNA condenses were observed (Figure 3). The cells that were suppressed from adhesion were more sensitive to apoptosis than free cells (Figure 4). DNA degradation examined by flow cytometry showed more apoptotic cells when the Raji cells were suppressed from adhesion.


Sodium butyrate induced DAPK expression and decreased the expression level of total FAK in Raji cells The results showed that the expression of DAPK increased, while that of total FAK decreased in the presence or absence of poly-HEME (Figure 5). It was suggested that sodium butyrate induced the expression of DAPK and then caused the protein levels of total FAK to decrease, prompting cell apoptosis. In addition, the results showed that the change of DAPK and FAK expression induced by sodium butyrate was identical in the presence or absence of poly-HEME.
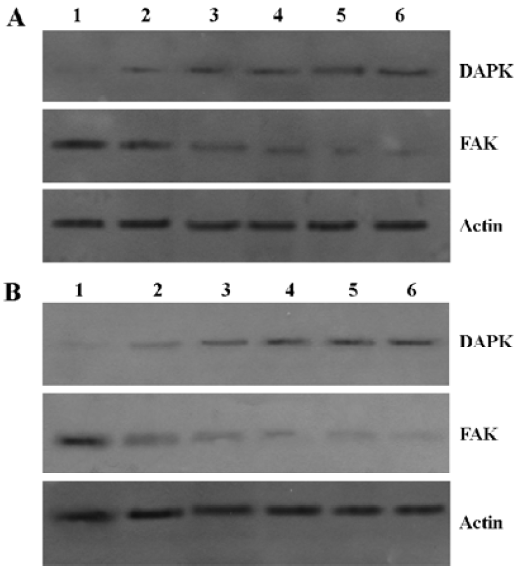
pcDNA3.1(+)-DCTP suppresses apoptosis induced by sodium butyrate It was found that pcDNA3.1(+)-DCTP reduced the pseudopod structure of Raji cells induced by 3 mmol/L sodium butyrate treatment for 4 d (Figure 6). In addition, we discovered that the Raji cells transfected with pcDNA3.1(+)-DCTP did not affect the protein levels of total DAPK and FAK in normal medium. However, it could suppress the decrease of the total FAK protein levels induced by sodium butyrate (Figure 7).


After being transfected with pcDNA3.1(+)-DCTP, cell apoptosis decreased (P<0.05). The apoptosis ratio of the pcDNA3.1(+)-DCTP transfected group was much lower than the pcDNA3.1(+) group (P<0.05) if cell adhesion was prevented. The apoptosis ratio of the Raji cells transfected with pcDNA3.1(+)-DCTP showed no significant differences between the polyHEME-treated and untreated groups. It was indicated that the pcDNA3.1(+)-DCTP group could resist the anoikis induced by sodium butyrate. On the contrary, the Raji cells transfected with pcDNA3.1(+) were susceptive to anoikis (P<0.05; Figure 8).

Discussion
Benjamin had indicated that sodium butyrate caused histone acetylation by suppressing the activity of histone deacetylase. Simultaneity, sodium butyrate induced promoter demethylation and reversed the methylation-mediated suppression of gene expression[3]. Similarly, we previously reported that sodium butyrate could induce demethylation of the DAPK gene promoter to inhibit cell growth. Our experiments proved that Raji cell growth was inhibited significantly when treated with sodium butyrate at the concentration of 0.75–6 mmol/L. Sodium butyrate trapped the cell cycle at the G0/G1 phase[8]. Recently, Zhang et al reported that histone deacetylase inhibitor TSA improved the chemotherapeutic efficacy of gastric carcinoma when treated in combination with anticancer drugs (5-FU, PTX, and SN38) by the upregulation of DAPK[15]. They indicated that the histone deacetylase inhibitor could induce cells to express DAPK and prompt cell apoptosis. The histone deacetylase inhibitor may be a potential chemotherapeutic drug.
The DAPK expression default causes tumor cells to lose sensibility to anoikis, so as to enable the anchor-independent survival of tumor cells[16]. Previous reports showed that the methylation of the DAPK gene promoter in Raji cells could silence DAPK expression. It would promote the apoptosis of Raji cells when the expression of DAPK was resumed in Raji cells[7,8]. Our results showed that sodium butyrate induced DAPK expression to promote Raji cell apoptosis.
DAPK is a multi-domain kinase located in myosin-II regulatory light chains (MLC). Through its cystoskeleton-binding region, DAPK phosphorylates MLC and changed the cell morphology[17].
It had been observed that the cells could have obvious morphological changes in DAPK-promoted HeLa cells and 293 cells. HeLa and 293 cells were induced to grow many fibrous pseudopod structures. There were many plate-type foot structures on the cell surface[18], which was in accordance with the results drawn by Kuo et al[19]. Our study showed that the rounded Raji cells grew into many fibrous pseudopod structures. The fibrous pseudopods converted the suspended growth to the adhesive growth of the Raji cells. Meanwhile, some cells underwent apoptosis when the cells adhered to the bottom of the culture flask. However, if cell adhesion was suppressed by polyHEME, the percentage of the apoptotic cells would increase. The results showed that the Raji cells induced by sodium butyrate became more susceptive to anoikis grown in an anchor-dependent manner.
Previous reports have shown that DAPK is an important apoptosis inducer[7,12–17]. Recently, Kuo et al reported that DAPK blocked migration and invasion independently of DAPK-induced apoptosis and DAPK regulated cell polarity to induce cell morphological change during migration, which may act together with its apoptotic function to suppress tumor progression[19]. It seemed that sodium butyrate induced DAPK expression in the Raji cells so as to cause change of cell morphology. In addition, Raji cells can resist DAPK-induced anoikis by adhesion on the culture flask. If the cells lost their ability to adhere onto the culture flask, they would undergo apoptosis.
Raveh et al reported that the expression of the C-terminal of DAPK suppressed the apoptosis that was promoted by DAPK. It was speculated that the C-terminal fragments suppressed the activity of DAPK by interacting with DAPK molecules. However, the precise mechanism has not been elucidated[20].
Kozak et al suggested that the translational initiation region should be in accordance with “A/GNNATGG” sequences in mRNA[21]. We screened the DAPK gene and identified 4231–4631 bp regions of coding sequence that contained 17 amino acid residues of C-terminal coding sequence tail of DAPK. There were “GGCATGG” sequences, which were a potential translational initiation region, according to Kozak et al’s theory, in 4231–4631 bp regions of coding sequence. Our previous study showed that pcDNA3.1(+)-DCTP did not affect the proliferation of human embryonic lung fibroblasts. However, pcDNA3.1(+)-DCTP could inhibit the toxicity of TNF-α[14].
Our results showed that the DAPK and FAK expression levels in the Raji cells transfected with pcDNA3.1(+)-DCTP was the same as normal Raji cells. It was suggested that pcDNA3.1(+)-DCTP did not affect the expression of DAPK and the protein levels of total FAK without sodium butyrate. In addition, pcDNA3.1(+)-DCTP did not affect sodium butyrate-induced DAPK expression. However, it could suppress the decrease of total FAK protein levels induced by sodium butyrate.
Our experiments also indicated that the Raji cells transfected with the pcDNA3.1(+)-DCTP were able to resist the apoptosis induced by sodium butyrate. In addition, the Raji cells, transfected with the pcDNA3.1(+)-DCTP, lost sensitivity to anoikis. The results demonstrated that DAPK induced anoikis together with a reduction in cell pseudopod quantity, indirectly implying that DAPK participated in cytomorphology change. However, Lebakken et al found that Raji cells transfected with the syndecan-1 gene could quickly stick to the matrix[22]. Obviously, there are other factors affecting the Raji cell cytomorphological change in addition to DAPK expression.
FAK participates in the signal conduction pathway of cell adhesion between cells and the extracellular matrix, and tightly combines some cell skeleton structure proteins, including fibronectin, laminin, actin, and fodrin. FAK is a survival protein that suppresses apoptosis and maintains cell suspended growth without anchor[23]. Wang et al found that DAPK could affect cell adhesion by changing the conformation of integrin. DAPK restrained the activity of integrin and blocked the cell survival signal induced by integrin. Simultaneously, it activated the apoptosis signal mediated by p53, disturbed the function of FAK, and downregulated the survival signal that FAK participated in[24]. Our experiment also demonstrated that sodium butyrate induced the Raji cells to restore DAPK expression, while the expression of FAK was downregulated in the Raji cells. DAPK expression degraded the survival signal of the cells and prompted the cells to die.
Wen et al showed that FAK was cleaved by caspases early in the apoptotic process. They demonstrated that Apo-2L and Fas induced the sequential proteolysis of FAK in Jurkat T cells. It was shown that the disruption of FAK contributed to the morphological changes observed in apoptotic suspension and adherent cells[25]. Kurenova et al also demonstrated that caspase-8 and the Fas-associated death domain(FADD)-dependent pathway mediated apoptosis by the attenuation of FAK expression in tumor cells[26]. It was well known that DAPK could activate the caspase-dependent pathway to promote cell apoptosis[16,17,24], so we thought that DAPK may mediate the decrease of the total protein of FAK by the caspase-dependent pathway in sodium butyrate-induced Raji cell apoptosis.
The Raji cells transfected with pcDNA3.1(+)-DCTP resisted the downregulation of the FAK protein and enabled the cells’ survival. We thought that pcDNA3.1(+)-DCTP expressed peptides to specifically suppress the DAPK activity, so the DAPK-mediated caspase-dependent apoptosis signal conduction pathway was reduced. The cells were able to maintain the total FAK protein level. Based on our experimental results, DAPK expression and the decrease of total FAK protein played a vital role in Raji cells susceptibility to anoikis.
In this paper we have shown that: (i) sodium butyrate induced DAPK expression and caused Raji cells to display many protrusions all around the cells; (ii) DAPK expression was an important reason to induce the suspended-growth Raji cells to adhere onto the culture flask; and (iii) DAPK expression prompted apoptosis by decreasing the total protein of FAK.
References
- Blottiere HM, Buecher B, Galmiche JP, Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc Nutr Soc 2003;62:101-6.
- Heerdt BG, Houston MA, Anthony GM, Augenlicht LH. Initiation of growth arrest and apoptosis of MCF-7 mammary carcinoma cells by tributyrin, a triglyceride analogue of the short-chain fatty acid butyrate, is associated with mitochondrial activity. Cancer Res 1999;59:1584-91.
- Benjamin D, Jost JP. Reversal of methylation-mediated repression with short-chain fatty acids: evidence for an additional mechanism to histone deacetylation. Nucleic Acids Res 2001;29:3603-10.
- Lee MG, Wynder C, Bochar DA, Hakimi MA, Cooch N, Shiekhattar R. Functional interplay between histone demethylase and deacetylase enzymes. Mol Cell Biol 2006;26:6395-402.
- Duan H, Heckman CA, Boxer LM. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t (14;18) lymphomas. Mol Cell Biol 2005;25:1608-19.
- Earel JK Jr, VanOosten RL, Griffith TS. Histone deacetylase inhibitors modulate the sensitivity of tumor necrosis factor–related apoptosis-inducing ligand-resistant bladder tumor cells. Cancer Res 2006;66:499-507.
- Kissil JL, Feinstein E, Cohen O, Jones PA, Tsai YC, Knowles MA, et al. DAP kinase loss of expression in various carcinoma and B-cell lymphoma cell lines: possible implications for role as tumor suppressor gene. Oncogene 1997;15:403-7.
- Zhang HT, Feng ZL, Liang NC, Zhu ZY, Ma JQ. Sodium butyrate induces Raji cells to express DAPK. Chin Pharmacol Bull 2005;21:1438-41.
- Follanan J, Moscona A. Role of cell shape in growth control. Nature 1978;273:345-9.
- Zhang HT, Wu J, Zhang HF, Zhu QF. Efflux of potassium ion is an important reason of HL-60 cells apoptosis induced by tachyplesin. Acta Pharmacol Sin 2006;27:1367-74.
- Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFκB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem 2004;279:6017-26.
- Zhang HT, Zhu ZY, Feng ZL, Li XY, Li MY, Ma JQ, et al. Effect of DAPK1 gene transfection on high-metastasis, non-small lung cancer cell PGCl3. Ai Zheng 2004;23:497-501.
- Zhang HT, Zhu ZY, Ji QM, Li XY, Li MY, Ma JQ. Cloning and sequence analysis of death associated protein kinase gene ORF and DAPK1 inducing Raji cell apoptosis. Chin Pathol Physiol J 2004;20:88-93. Chinese..
- Zhang HT, Feng ZL, Zhu ZY, Ma JQ, Liang NC. Effect on TNF-α inhibit human embryonic lung fibroblast proliferation by death associated protein kinase carboxylic terminal peptide. Basic Clin Med 2006;26:975-9. Chinese..
- Zhang X, Yashiro M, Ren J, Hirakawa K. Histone deacetylase inhibitor, trichostatin A, increases the chemosensitivity of anticancer drugs in gastric cancer cell lines. Oncol Rep 2006;16:563-8.
- Inbal B, Cohen O, Polak-Charcon S, Kopolovic J, Vadai E, Eisenbach L, et al. DAP kinase links the control of apoptosis to metastasis. Nature 1997;390:180-4.
- Cohen O, Feinstein E, Kimchi A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J 1997;16:998-1008.
- Bialik S, Bresnick AR, Kimchi A. DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ 2004;11:631-44.
- Kuo JC, Wang WJ, Yao CC, Wu PR, Chen RH. The tumor suppressor DAPK inhibits cell motility by blocking the integrin-mediated polarity pathway. J Cell Biol 2006;172:619-31.
- Raveh T, Berissi H, Eisenstein M, Spivak T, Kimchi A. A functional genetic screen identifies regions at the C-terminal tail and death-domain of death-associated protein kinase that are critical for its proapoptotic activity. Proc Natl Acad Sci USA 2000;97:1572-7.
- Kozak M. The scanning model for translation: an update. J Cell Biol 1989;108:229-41.
- Lebakken CS, Rapraeger AC. Syndecan-1 mediates cell spreading in transfected human lymphoblastoid (Raji) cells. J Cell Biol 1996;132:1209-21.
- Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/akt in regulating fibroblast survival in response to contraction of type i collagen matrices via a (beta) 1 integrin viability signaling pathway. J Biol Chem 2004;279:33024-34.
- Wang WJ, Kuo JC, Yao CC, Chen RH. DAP-kinase induces apoptosis by suppressing integrin activity and disrupting matrix survival signals. J Cell Biol 2002;159:169-79.
- Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem 1997;272:26056-61.
- Kurenova E, Xu LH, Yang X, Baldwin AS, Craven RJ, Hanks SK, et al. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol Cell Biol 2004;24:4361-71.
