Antisense oligonucleotide targeting p53 increased apoptosis of MCF-7 cells induced by ionizing radiation1
Introduction
Ionizing radiation (IR) is widely used in the therapy of solid tumours, with more than half of all cancer patients receiving radiation therapy (RT) during the course of treatment. After surgery, RT is now proved to be the most powerful medical treatment in the fight against cancer, especially for localized disease that has not spread. Rational and effective use of its killing power depends on understanding IR-mediated responses at the molecular, cellular and tissue levels. It is known that irradiation is used to treat virtually all types of solid malignancies, but to varying degrees of success. That is, some tumors are highly responsive to low doses of radiation (eg, lymphomas, seminomas), and other tumors are typically very radioresistant and tend to progress even after high radiation doses (eg, melanoma, glioblastoma)[1]. The reasons for such radioresistance in those tumors are multiple and varied.
Nowadays, the main focus of radiation-related studies has switched to the analysis of molecular mechanisms of the IR-induced cellular response. With the hope that increased understanding would facilitate the development of new strategies for overcoming the radioresistance. Accumulated evidence demonstrate that p53 is an important molecule for determining cell radiosensitivity[2]. It is reported that the degree of radiation-induced apoptosis has been shown to correlate with the p53 wild-type (wt) status[3]. Furthermore, apoptosis is induced when wt-p53 is transfected into certain cell lines lacking p53[4,5]. It is also known that several downstream genes of p53, such as p21, cyclin G, and GADD45, whose expression products function as regulators of diverse aspects of cell growth, is rapidly upregulated after ionizing radiation-induced DNA damage[6–8]. The induction of the p21 protein after ionizing radiation mediates p53-dependent G1 arrest via its inhibitory effects on cyclin-dependent kinase (CDKs) required for S-phase entry[9]. Recently, it is believed that cell cycle phase is an important factor determining cell radiosensitivity[10,11]. Proper cell cycle arrest may allow cells the time to repair DNA damage, which is thought to be associated with cell survival in response to radiation. This suggests that p53 can not only induce cell apoptosis, but also plays a role in protecting cells against apoptosis by regulating the progression through the cell cycle when cells respond to irradiation.
Because of the complexity of p53 function, a problem arises as to whether wt p53 functions as a cell apoptotic activator or not when responding to radiation. There are many published reports of investigations of the relationship between p53 mutations and radiosensitivity in vitro and in animals[12–16]; however, the role of p53 in determining sensitivity to radiotherapy is still controversial. Böhnke et al[17] showed that p53 wt cells (MCF-7, MCF-BB, LNCaP, etc) were on average 1.3-times more radiosensitive than mutant cells (DU-145, RT112, and SCC4451), suggesting that wt p53 may mainly serve as a pro-apoptotic gene to increase radiosensi-tivity. However, this result was obtained mainly through statistical analysis, and might be limited to reflect the truth. Further study is needed on this.
Antisense compounds are useful tools for biological research and can reduce gene expression by efficient annealing of complementary sequences to the target mRNA[18,19]. The ability to suppress the expression of individual genes with a high degree of specificity could distinguish antisense therapy from other modes of treatment. Therefore, the aim of our study is to identify antisense compounds targeting p53 and clarify its effect on radiosensitivity in MCF-7 cells.
Materials and methods
Cell line and culture DU-145 and MCF-7 cells were obtained from Chinese Academy of Medical Science (Beijing, China). Cells were cultured in DMEM medium (Invitrogen, San Diego, CA, USA) containing 10% fetal bovine serum (GIBCO BRL, Grand Island, NY, USA), 100 kU/L penicillin and 100 mg/L streptomycin. All cultures were incubated at 37 ºC in a 5% carbon dioxide (CO2) atmosphere.
Radiation Cells after transfection for 24 h were irradiated with 5 Gy IR by using a 60Co irradiator at a dose-rate of about 2.5 Gy/min for 2 min.
Antisense oligodeoxynucleotides Antisense sequences used in these experiments were designed by a computational neural network mode and mfold server[20,21]. BLAST confirmed they were specific for the human p53 mRNA. 20 mer antisense ODNs were synthesized to about 99% (MGW Biotech, Ebersberg, Germany). p53 antisense oligodeoxy-nucleotides (AS) sequences corresponding to different regions of human p53 mRNA were as follows:
AS1: 5'-ACG CTC CCA GCC CGA ACG CA-3' (bases 169 to188)
AS2: 5'-TGG CGC GGA CGC GGG TGC CG-3' (bases 710 to 729)
AS3: 5'-CAC CAC CAC ACT ATG TCG AA-3' (bases 886 to 905)
AS4: 5'-TCC CCA TCC TCC TCC CCA CA-3' (bases 1615 to 1634)
AS5: 5'-AAA GTT TTA TTG TAA AAT AA-3' (bases 2603 to 2622).
Lip-mediated transfection of antisense oligodeoxynucle-otides Cells were plated in 6-well plates at a density of 1×105 cells per well. Transfections were carried out after plating for 24 h when cells reached a confluence of 50%–80%. Lipofectamine reagent (Invitrogen) was used for transfection in this experiment, and performed according to the manufacturer. Antisense ODNs concentrations were selected as 0.2 µmol/L, 0.4 µmol/L, and 0.8 µmol/L respectively. After transfection (incubation for 6 h at 37 ºC), cells were washed with PBS and incubated in fresh culture medium until additional analyses in this experiment.
Cell viability The effects of AS on cellular viability were determined using MTS assay. Briefly, 3×103 cells were seeded in 96-well microtiter plate and allowed to attach overnight. Cells were then transfected with different antisense ODNs concentrations (0.2 µmol/L, 0.4 µmol/L, and 0.8 µmol/L). After transfection for 24 h, samples were treated with 5 Gy IR. At post-irradiation 24 h, 20 μL of MTS (Sigma, St Louis, MO) was added to each well. Then the 96-well microtiter plate was incubated at 37 ºC for 2 h, and 490 nm absorbance value was determined using a MR 600 Microplate reader (Wallac 1420 Multilabel counter). Each assay was performed in quadruplicate. Cell survival rate (%) is calculated as: [1-(Acontrol-Asample)/(Acontrol-Ablank)]×100
RT-PCR After transfection with AS for 48 h, total RNA was extracted using TRIzol (Invitrogen) from MCF-7 cells as the manufacturer' s instruction (Qiagen, Chatsworth, CA, USA). RNA was obtained from both untransfected MCF-7 cells and cells transfected with 0.4 µmol/L AS. RT-PCR was carried out by using 2 µg of RNA for the RT reaction (as per the manufacturer' s instruction, Qiagen). p53 and Bax primers toward human p53 and Bax gene were then used in the PCR to measure p53 RNA levels (5'-GAATCTCCGCAA-GAAAGG-3', forward; 5'-TGGGCATCCTTGAGTTCC-3', reverse) and Bax RNA level (5'-GTTGTCGCCCTTTTCTA-CTT-3', forward; 5'- TCAGCCCATCTTCTTCCA-3', reverse), respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 5'-ACCACAGTCCATGCCATCAC-3' (forward) and 5'-TCCACCACCCTGTTGCTGTA-3' (reverse) were used on the same RT reaction as a quantitative control for PCR. Briefly, first strand cDNA was synthesized using a Oligo(dT)15 primer at 42 ºC for 2 h. PCR reaction for p53 and Bax were performed following 30 cycles of denaturing at 95 ºC for 30 s, annealing at 55 ºC, and 50 ºC for 30 s, respectively, and extending at 72 ºC for 30 s.
Western-blot analysis Cells were lysed in RIPA buffer [10 mmol/L Tris-HCl (pH 7.4), 1% deoxycholate, 1% NP 40, 150 mmol/L NaCl, 0.1% SDS, 0.2 mmol/L phenylmethyl sulfonyl fluoride, 1 µg/mL aprotinin and 1µg/mL leupeptin] for 30 min on ice. The lysates were centrifuged at 15 000×g for 15 min to remove debris. Proteins sample (30 μg) were separated by 12% SDS-PAGE gel and transferred onto PVDF membranes (Hybond-polyvinylidene difluoride membranes, Amersham Biosciences). p53 and Bax protein was identified using anti-p53 or anti-Bax primary and peroxidase-conjunct secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Finally, the reactive band was visualized by an ECL-plus Detection Kit (Amersham Biosciences) and scanned by Gel Doc 1000 (Bio-Rad).
Cell cycle and apoptosis analysis Cell cycle assays were performed as described previously[22]. Briefly, cells were harvested at 24 h after IR, and fixed with 70% ethanol at -20 ºC overnight. The fixed cells were washed twice again with PBS, and stained with 50 μg/mL propidium iodide (PI) in the presence of RNase A. The stained cells were analyzed for DNA content by fluorescence-activated cell sorting (FACS) in a FACScan (SOBR model, Becton Dickinson Instrument, San José, CA, USA). Cell cycle fractions were quantified with CellQuest (Becton Dickinson). For cell apoptosis analysis, MCF-7 cells were examined by annexin V-propidium iodide staining in conjunction with FACs analysis. Briefly, MCF-7 were plated and either mock-treated or irradiated with 5 Gy IR. After 24 h, both untreated and irradiated MCF-7 was incubated with annexin V-fluorescein isothiocyanate (FITC, BAOSAI Biosea Biochnology, Beijing, China) following the manufacturer’s specifications. Binding of FITC-conjugated annexin V and propidium iodide (PI) was analyzed by flow cytometry.
Statistics Data were expressed as mean ± SD. Statistical analysis was carried out using Student’s t-tests (two-tailed). P<0.05 indicates statistical significance.
Results
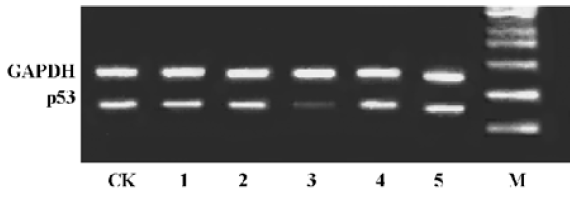
Effect of antisense ODNs on p53 mRNA level We firstly obtained five antisense oligonucleotides (AS) targeting p53 using AO predict tool based on a neural network. To evaluate expression of p53 mRNA in MCF-7 cells, RT-PCR was performed on extracted mRNA aliquots from cells. Of the five AS synthesized, only AS3 markedly downregulated p53 mRNA level in MCF-7 cells after transfection with the aid of Lipofectamine reagent (Figure 1). Therefore, AS3 was used to analyze the effect of AS targeting p53 on radiosensitivity in cells in the following experiments.
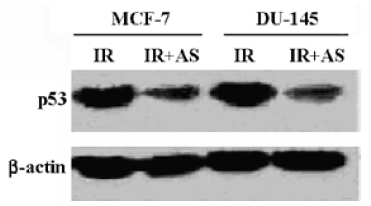
Effects of AS3 on p53 protein expression In order to analyze the effect of AS3 on p53 protein content in cells, AS3 were transfected into MCF-7 and DU-145 cells before irradiation. At 24 h after transfection of AS3, both MCF-7 and DU-145 cells were treated with 5-Gy irradiation. The total proteins were extracted at 24 h after irradiation. In this experiment, it was observed that AS transfer led to a remarkable decline of p53 protein level in both MCF-7 and DU-145 cells at 24 h after irradiation (Figure 2).
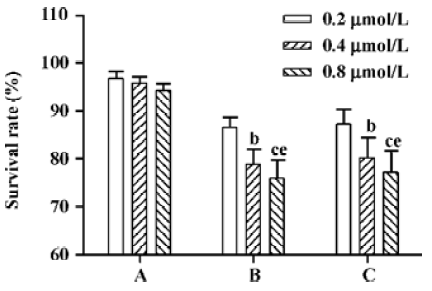
Effects of AS3 on cell viability after IR For examination of effect of AS3 targeted to p53 on the radiosensitivity in DU-145 and MCF-7 cells, AS3 was added to both cells at final concentrations of 0.2, 0.4, and 0.8 µmol/L before irradiation or at 6 h after irradiation. From Figure 3, it was clear to observe that transfection of AS3 before irradiation had little effect on cell survival in DU-145 cells at 24 h after IR. However, survival rate of MCF-7 cells decreased significantly at 24 h, and its inhibitory effects exhibited a dose-dependent manner, suggesting that transfection of AS3 before radiation can increase MCF-7 cells radiosensitivity to certain extent (Figure 3). To examine whether AS3 transfer after IR still increased radiosensitivity of MCF-7, we added the AS3 to MCF-7 cells at 6 h after treatment with 5-Gy. Interestingly, transfection of AS3 after irradiation also increased MCF-7 cells radiosensitivity in this experiment.
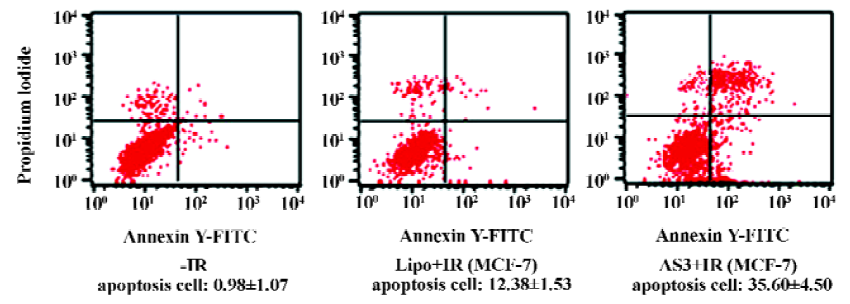
Effects of AS3 on cell cycle distribution and cell apoptosis Flow cytometry was then used to quantify changes in the cell apoptosis at 24 h after IR. As shown in Figure 4, it was clearly observed that IR induced slight apoptosis in MCF-7 cells. It is noted that transfection of AS3 induced marked apoptosis in MCF-7 cells. To examine the effect of AS3 on cell-cycle progression, MCF-7 with wt p53 was transfected with AS3. At 24 h after IR, cells were fixed at -20ºC overnight, and then fixed cells were stained with 50 µg/mL propidium iodide (PI) in the presence of RNase and analyzed cell cycle progression. Cell-cycle redistribution was shown in Table 1 and Figure 5. Transfection of AS3 decreased the fraction of G0–G1 phase cell from 60.38%± 1.07% to 45.94%±1.53%, suggesting that AS3 abrogated G1 phase arrest to certain extent by inhibition of p53 expression (Table 1). In compari-son, S phase cells increased from 20.42%±2.33% to 28.82%±1.68%. It is interesting to observe that transfection of AS3 increased G2 phase cells from 19.19%±1.15% to 25.24%±2.46%.
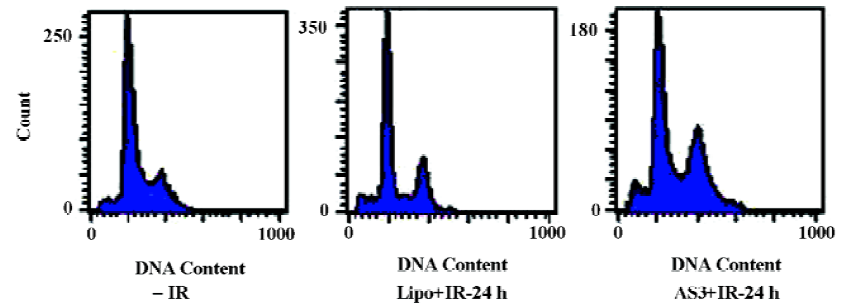
Full table
Effects of AS3 transfer on Bax mRNA level It is known that p53 protein is a positive regulator of the proapoptotic gene Bax transcription. However, in this experiment, inhibition of p53 expression had little effect on Bax mRNA level and protein content measured in MCF-7 cells at 24 h after IR (Figure 6). This result suggests that the increased apoptosis after transfection with AS3 in MCF-7 cells may not be associated the Bax gene expression.
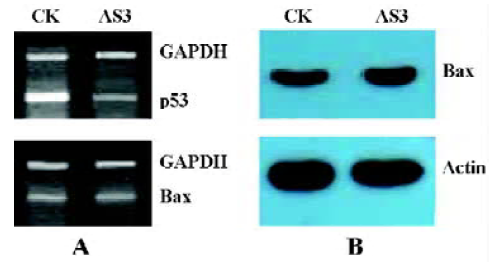
Discussion
In this study, we firstly designed antisense sequences using a neural network. Furthermore, in order to improve the predicted effective antisense ODNs, the p53 mRNA secondary structure was also predicted according to Zuker[21]. Based on the two results, we obtained several candidate sequences. Among those, AS3 was identified to successfully suppress p53 mRNA and protein level in MCF-7 and DU-145 cells (Figure 1 and 2). The results indicated that computational prediction is a suitable method to find putative target sites.
To investigate the effect of AS3 on cell radiosensitivity, we selected MCF-7 cells with wt p53 and DU-145 cells with mutant p53. It is believed that loss of p53 protein function caused by mutation or deletion results in ability of anti-apoptosis in cell[17,23,24]. However, in this experiment, down-regulation of wt p53 using antisense compounds leads to increase of apoptosis in MCF-7 cells after irradiation (Figure 4). It is well known that loss of p53 function decreases the apoptotic response in several cell types after irradiation programmed for proliferation-independent early-interphase apoptosis[25,26]. However, in most cell types of solid tumors, early apoptosis after irradiation before the release from G2 block is not a general phenomenon in all mammalian cells. A prerequisite for radiation-induced late apoptosis is cell-cycle progression beyond the radiation-induced G2 phase block. The apoptosis in irradiated cells in our experiment occurred mainly after 24 h, which belongs to IR-induced late apoptosis. This kind of apoptosis may be closely related to cell-cycle progression beyond the radiation-induced cell-cycle arrest.
Presently, it is generally believed that cell phase is crucial for determining cell radiosensitivity. The cell-cycle checkpoints pathway has been of interest to the field of irradiation biology because it is related to the radiosensitivity of cancer. Such checkpoint mechanisms allow the cell time to repair the DNA damage before cell-cycle progression is resumed. In this experiment, the fraction of G1 phase cells decreased significantly in MCF-7 transfected with AS3 at 24 h after IR (Table 1). Several studies indicated that increasing radiosensitivity by transfection of antisense oligonucleotide targeting p21 is associated with G1 cell arrest block[27–29]. There-fore, abrogation of G1 cell arrest through inhibition of p53 gene expression might be an important cause for the increase of radiosensitivity in MCF-7 cells. In fact, Sak et al[30] reported that antisense oligonucleotide targeting p53 transfer increased A549 (wt p53) apoptosis induced by IR, and decreased the fraction of G1 and G2 phase cells. However, in our experiment, we did not observe AS3 transfer decreased G2/M phase cell at 24 h after IR. In contrast, a slight increase of G2/M phase cells was observed in MCF-7 cells. Interestingly, AS3 transfection after IR also increased MCF-7 cells radiosensitivity. In addition to regulation of cell-cycle checkpoints that arrest cells, giving them time to repair damage, p53 also activates genes that are in some way more directly involved in DNA repair responses[31]. However, it is believed that IR only induced p53-dependent DNA repair not but p53-dependent apoptosis under low dose radiation[32]. We therefore speculate that the increased radiosensitivity by AS3 transfer after IR may be associated with down-regulation of DNA repair related genes expression.
In conclusion, antisense ODNs targeting against p53 transfer inhibit p53 gene expression efficiently. The inhibition of p53 abrogated G1 phase arrest to a certain extent, and increased MCF-7 cell radiosensitivity. We speculated the increase of radiosensitivity might be associated with abrogation of cell arrest. Moreover, the developed efficient antisense ODNs may be used as tools for continuative investigations with regard to the role of p53 in tumor events.
References
- Jung M, Dritschilo A. Signal transduction and cellular responses to ionizing radiation. Semin Radiat Oncol 1996;6:268-72.
- Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev 2003;3:117-28. (cancer).
- Fei P, Bernhard EJ. EI-Deiry WS. Tissue-specific induction of p53 targets in vivo. Cancer Res 2002;62:7316-27.
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited interleukin-6. Nature 1991;352:345-7.
- Yonish-Rouach E, Grunwald D, Wilder S, Kimchi A, May E, Lawrence JJ. p53-mediated cell death: relationship to cell cycle control. Mol Cell Biol 1993;13:1415-23.
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM. WAF1, a potential mediator of p53 tumour suppression. Cell 1993;75:817-25.
- Reimer CL, Borras AM, Kurdistani SK, Garreau JR, Chung M, Aaronson SA. Altered regulation of cyclin G in human breast cancer and its specific localization at replication foci in response to DNA damage in p53+/+ cells. J Biol Chem 1999;274:11022-9.
- Chin PL, Momand J, Pfeifer GP. In vivo evidence for binding of p53 to consensus binding sites in the p21 and GADD45 genes in response to ionizing radiation. Oncogene 1997;15:87-99.
- Bow JJ, Howdgson B. Replication licesing-defining the proliferative state? Trends cell Biol 2002;12:72-8.
- Short SC, Woodcock M, Marples B, Joiner MC. Effects of cell cycle phase on low-dose hyper-radiosensitivity. Int J Radiat Biol 2003;79:99-105.
- Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys 2004;59:928-42.
- Ribeiro JCC, Barnetson AR, Fisher RJ, Mameghan H, Russell PJ. Relationship between radiation response and p53 status in human bladder cancer cells. Int J Radiat Biol 1997;72:11-20.
- Kawashima K, Mihara K, Usuki H, Shimizu N, Namba M. Transfected mutant p53 gene increases X-ray-induced cell killing and mutation in human fibroblasts immortalized with 4-nitroquinoline 1-oxide but does not induce neoplastic transformation of the cells. Int J Cancer 1995;61:76-9.
- Biard DSF, Martin M, Rhun YL, Duthu A, Lefaix JL, May E. Concomitant p53 gene mutation and increased radiosensitivity in rat lung embryo epithelial cells during neoplastic development. Cancer Res 1994;54:3361-4.
- McIlwrath AJ, Vasey PA, Ross GM, Brown R. Cell cycle arrests and radiosensitivity of human tumor cell lines: dependence on wild-type p53 for radiosensitivity. Cancer Res 1994;54:3718-22.
- Brachman DG, Beckett M, Graves D, Haraf D, Vokes E, Weichs-elbaum RR. p53 mutation does not correlate with radiosensitivity in 24 head and neck cancer cell lines. Cancer Res 1993;53:3667-9.
- Böhnke A, Westphal F, Schmidt A, El-Awady A, Dahm-Daphi J. Role of p53 mutations, protein function and DNA damage for the radiosensitivity of human tumour cells. Int J Radiat Biol 2004;1:53-63.
- Dai JM, Zhang SQ, Zhang W, Lin RX, Ji ZZ, Wang SQ. Antisense oligodeoxynucleotides targeting the serine/threonine kinase Pim-2 inhibited proliferation of DU-145 cells. Acta Pharmacol Sin 2005;26:364-8.
- Yang SP, Song ST, Tang ZM, Song HF. Optimization of antisense drug design against conservative local motif in stimulant secondary structures of HER-2 mRNA and QSAR analysis. Acta Pharmacol Sin 2003;24:897-902.
- Chalk AM, Sonnhammer EL. Computational antisense oligo prediction with a neural network model. Bioinformatics 2002;18:1567-75.
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003;31:3406-15.
- Shao RG, Cao CX, Shimizu T, O’Connor PM, Kohn KW, Pommier Y. Abrogation of an S-phase checkpoint and potentiation of camptothecin cytotoxicity by 7-hydroxystaurosporine (UCN-01) in human cancer cell lines, possibly influenced by p53 function. Cancer Res 1997;57:4029-35.
- Matsuzoe D, Hideshima T, Kimura A, Inada K, Watanabe K, Akita Y, et al. p53 mutations predict non-small cell lung carcinoma response to radiotherapy. Cancer Lett 1999;135:189-94.
- Nicole C, Christa Z, Margit S, Ingrid S, Dan T, Uwe W, et al. p53-dependent radioresistance in ovarian carcinoma cell lines. Cancer Lett 2000;150:191-9.
- Slichenmer WJ, Nelson WG, Slebos RJ, Kastan MB. Loss of a p53-assocaited G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res 1993;53:4164-8.
- Brachman DG, Beckett M, Graves D, Haraf D, Vokes E, Weichselbaum RR. p53 mutation does not correlate with radiosensitivity in 24 head and neck cancer cell lines. Cancer Res 1993;53:3667-9.
- Liu XF, Xia YF, Li MZ, Wang HM, He YX, Zheng ML, et al. The effect of p21 antisense oligodeoxynucleotides on the radiosensitivity of nasopharyngeal carcinoma cells with normal p53 function. Cell Biol Int 2006;30:283-7.
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995;82:675-84.
- Kokunai T, Urui S, Tomita H, Tamaki N. Overcoming of radioresistance in human gliomas by p21WAF1/CIP1 antisense oligonucleotide. J Neurooncol 2001;51:111-9.
- Sak A, Wurm R, Elo B, Grehl S, Christoph P, Stuben G, et al. Increased radiation-induced apoptosis and altered cell cycle progression of human lung cancer cell lines by antisense oligodeoxy-nucleotides targeting p53 and p21WAF1/CIP1. Cancer Gene Ther 2003;10:926-34.
- El-Deiry WS. The role of p53 in chemosensitivity and radio-sensitivity. Oncogene 2003;22:7486-95.
- Li G, Ho VC. p53-dependent DNA repair and apoptosis respond differently to high-and low-dose ultraviolet radiation. Br J Dermatol 1998;139:3-10.
