Metallothionein mediates cardioprotection of isoliquiritigenin against ischemia-reperfusion through JAK2/STAT3 activation
Introduction
Myocardial infarction is one of the major causes of death in many countries. Although restoration of blood flow is the only way to save the myocardium from necrosis, reperfusion-induced injuries with the help of thrombolytic therapy are at the background of a high mortality rate[1]. Extensive studies show that myocardial ischemia-reperfusion (MI/R) injury is associated with increased generation of reactive oxygen species (ROS). These oxygen free radicals may result in depressions in contractile function, arrhythmias, depletion of endogenous antioxidant network, and membrane permeability changes[2].
In recent years, accumulating experiments have shown that metallothionein (MT), a low molecular non-enzyme protein, is a strong endogenous cytoprotective agent against cardiovascular injury. It plays an important role in detoxifying against harmful heavy metals, scavenging oxygen radicals, and stabilizing biomembranes[3]. The antioxidant function of MT was first suggested in the early 1980s. Studies in vitro have revealed that MT reacts directly with reactive oxygen species, including superoxide, hydroxyl, and hydrogen peroxide. In particular, the antioxidant function of MT in the heart has been explored extensively. The data gathered from recent studies using a cardiac-specific, MT-overexpressing transgenic mouse have provided direct evidence to support this physiological role of MT[4]. Under acute and chronic oxidative stress conditions such as treatment with doxorubicin, ischemia-reperfusion, and dietary copper-induced restriction, MT-overexpressing transgenic mouse hearts displayed a marked resistance to the injurious consequences, including biochemical, pathological, and functional alterations. This protective action of MT correlates with its inhibition of reactive oxygen species-induced lipid peroxidation[5]. Exogenous administration with MT or induction of endogenous MT production may be used as a new strategy in prevention and therapy of cardiovascular diseases[6,7].
The janus kinase/signal transducer and activator of transduction (JAK/STAT) pathway is a newly discovered intracellular signal-transducing pathway that is activated by oxygen radicals, various cytokines, and growth factors in ischemic heart diseases. Recent studies have demonstrated that MI/R induce rapid activation of this pathway[4]. Although the functional consequences of this event remains to be elucidated, there is emerging evidence that JAK/STAT signaling plays an important role in the development of the cardioprotected phenotype associated with ischemic precon-ditioning.
Some studies in vivo also indicated that activation of STAT 3 protects myocardium from ischemia-reperfusion injury at least partially through metallothionein, including MT-1 and MT-2[8]. Specifically, brief episodes of MI/R activated JAK 1 and JAK 2, followed by recruitment of STAT 1 and STAT 3, resulting in transcriptional upregulation of MT[9,10].
Flavonoids are ubiquitous compounds found in a wide variety of edible plants, fruits, vegetables, grains and red wine. They are an integral part of the human diet, and are important and effective constituents of some medicines, especially of Chinese herbal medicines[11]. Epidemiological studies have indicated an association between the consumption of flavonoids and a reduced incidence of diseases such as stroke[12], coronary heart diseases[13] and cancer[14]. Glycyrrhiza glabra (family: Leguminosae), the licorice plant, is widely used in Chinese medicine and food and has a history of consumption for the past 6000 years. Isoliquiritigenin (ISL), 2',4',4'-three hydroxy chalcone, one of the components in the root of Glycyrrhiza glabra, is a member of the flavonoids, which have shown various biochemical activities, such as vasorelaxant effect, antioxidant, anti-platelet, anti-tumor, anti-allergic, antiviral activities and estrogenic properties[15]. However, the effects of ISL on MI/R injury have not been studied. Therefore, the protective effects of ISL and its molecular mechanism were investigated by using coronary artery occlusion–reperfusion rat model in vivo, which is considered to more closely mimic the clinical situation.
Materials and methods
Reagents The roots of Glycyrrhiza glabra used in this study were collected in Yili, Xinjiang Province, China and authenticated by Dr Ming-xi JIANG, Wuhan Institute of Botany, Chinese Academy of Sciences. The extraction and identification of ISL are identical with our description in a previous study[16]. ISL was finally suspended in a 0.5% carboxymenthylcellulose (CMC)-saline solution. Creatinine phosphokinase (CPK) and lactate dehydrogenase (LDH) assay kits were from Jian Cheng Biology Research Institute (Nanjing, China). The antibodies for MT-1, MT-2, rabbit anti-JAK 2, anti-STAT 1, anti-STAT 3, anti-ERK 1, anti-ERK 2, and anti-Akt polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phospho-JAK 2, STAT 1, STAT 3, ERK 1, ERK 2, and Akt were from New England Biolabs (Beverly, MA, USA). RNA polymerase chain reaction (PCR) kit (AMV) was purchased from TaKaRa Biotechnology (Dalian, China). All other chemicals were reagents of molecular biology grade obtained from standard commercial sources. AG490 was purchased from Calbiochem-Novabiochem (La Jolla, CA, USA).
Animals and treatment Male Sprague-Dawley rats (Grade II, Certificate N
Surgical preparation Rats of all experimental groups were anaesthetized intraperitoneally with pentobarbitone sodium (60 mg/kg). The neck was opened with a ventral midline incision, and a tracheotomy was performed, the rats were ventilated with room air from a positive pressure ventilator (Crompton Parkinson, Doncaster, Yorkshire, UK). The right carotid artery was cannulated with a polyethylene tube to obtain the blood and extract the serum used for assaying the activities of LDH and CPK at the end of surgery protocol. A left thoracotomy was performed at the fifth intercostals space and the pericardium was opened to expose the heart. The left arterial descending coronary artery (LAD) was ligated 2 mm from its origin by a 5–0 silk suture with a traumatic needle and ends of this ligature were passed through a small vinyl tube to form a snare. The thoracic cavity was covered with saline-soaked gauze to prevent the heart from drying. The animals were allowed to stabilize for 10 min before LAD ligation. Myocardial ischemia was induced by one stage occlusion of the LAD by pressing the polyethylene tubing against the ventricular wall and then fixing it in place by clamping the vinyl tube with a hemostat. Electrocardiographic leads were attached subcutaneous electrodes to monitor limb lead II. The animals then underwent 15 min of ischemia, confirmed visually in situ by the appearance of regional epicardial cyanosis and ST-segment elevation. The myocardium was reperfused by releasing the snare gently for a period of 2 h[17]. A lead II electrocardiogram was monitored throughout the study. At the end of reperfusion period, rats were killed for biochemical studies and other analysis.
Evaluation of myocardial infarct size The frozen heart tissue samples were cut into 2-mm transverse slices. The slices were incubated in 1% triphenyl tetrazolium chloride (TTC) in pH 7.4 buffer at 37 oC for 20 min. TTC stains living tissue a deep red color while necrotic tissue is TTC negative and is tan in color. The infarct and risk zone considered to be the area lacking fluorescence under UV light was traced.
Determination of arrhythmias Before and during ischemia and reperfusion periods, ECG changes were recorded. The incidences and duration of ventricular premature beats (VPB), ventricular tachyarrhythmia (VT), and ventricular fibrillation (VF) in surviving animals were determined.
Measurement of plasma LDH and CPK activities The blood samples drawn from the carotid artery at the end of MI/R period were collected in heparinized tubes. These samples were kept at 4 oC until they were centrifuged at 2000×g for 15 min. The plasma was recovered and aliquots were used for determination of LDH and CPK activities with commercial kits.
Reverse transcription PCR analysis Total RNA and then cDNA were prepared from rats myocardial tissues using TRIzol RNA extraction and RT-PCR kits. cDNA synthesis, semi-quantitative RT-PCR for MT-1, MT-2, cyclooxygenase-2 (COX-2), iNOS and GAPDH mRNA, and the analysis of the results obtained were performed as described in a previous study[18]. The sense and anti-sense primer sequences used were: MT-1: 5'-GGTCTTCTCTGTTGGGGACA-3', 5'-GCTG G-GTTGGGTTGGTCCGATACTA-3'; MT-2: 5'-TAGATGGA-TCCTGCTCCTGC-3', 5'-CACTTGTAGGAAGCCCTCTT-3'; COX-2: 5'-CCATGTCAAAACCGTGGTGAATG-3', 5'-AGTG-GAGTTGGGCAGTCATCAG-3'; iNOS: 5'-GTGAGGATCAA-AACTGGGG-3', 5'-ACCTGCAGGTTGGACCACTG-3'; GAPDH: 5'-CCTCTATGCCAACACAGT-3',5'-AGCCACC-AATCCACACAG-3'. These primers set yielded PCR products of 145, 53, 374, and 200 bp for MT-1, MT-2, and GAPDH, respectively. PCR (26 cycles for MT-1, MT-2 cDNA, 35 cycles for COX-2 cDNA, 32 cycles for iNOS cDNA, and 23 cycles for GAPDH cDNA) was performed using the GeneAmp PCR System 2400 (Perkin Elmer Life and Analytical Sciences, Boston, MA, USA). The PCR products were electrophoresed through a 1.5% agarose gel and visualized by ethidium bromide staining and UV irradiation.
Western blot analysis Left ventricles from the hearts were homogenized in a buffer containing 25 mmol/L Tris-HCl, 25 mmol/L NaCl, 1mmol/L orthovanadate, 10 mmol/L pyrophosphate, 10 mmol/L okadaic acid, 0.5 mmol/L EGTA, and 1 mmol/L PMSF. Protein (100 µg) of each heart homogenate was incubated with 1 μg of antibody against MT-1, MT-2, JAK 2, STAT 1, STAT 3, ERK 1, ERK 2, and Akt for 1 h at 4 oC. The immune complexes were precipitated with protein A Sepharose, immunoprecipitates separated by SDS-PAGE and immobilized on polyvinylidene difluoride membrane. The membrane was immunoblotted with PY20 to evaluate the phosphorylation of JAK 2, STAT 1, STAT 3, ERK 1, ERK 2, and Akt. The membrane was stripped and reblotted with specific antibodies against MT-1, MT-2, JAK 2, STAT 1, STAT 3, ERK 1, ERK 2, and Akt. The resulting blots were digitized and subjected to densitometric scanning using a standard NIH image program.
Statistic analysis Results of all the above estimations have been indicated in terms of mean±SD. The difference between means was analyzed by ANOVA test. Minimum level of significance was fixed at P<0.05.
Results
Effects of ISL on myocardial infarction The myocardial infarction induced by ischemia-reperfusion was measured by TTC staining. As compared with the vehicle group, the percentage of myocardial infarct size was obviously decreased in the ISL 20 mg/kg group from 50.91%±1.90% to 20.45%±1.03% (P<0.01) (Table 1). Pretreatment with tyrphostin AG490 significantly blocked the anti-infarct effect of ISL.
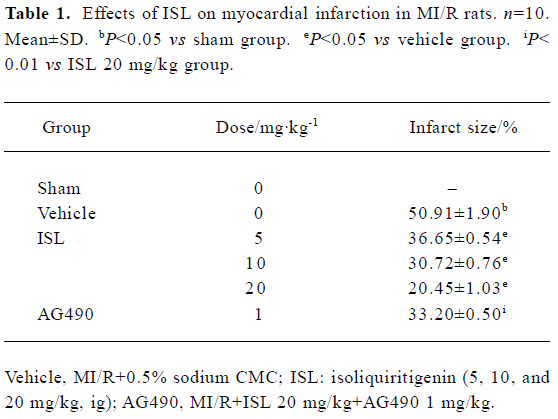
Full table
Effects of ISL on ischemia-reperfusion arrhythmias There was no significant difference between vehicles and rats treated with ISL with respect to heart rate (HR) immediately prior to coronary occlusion. These were 435±6 and 423±8 beats/min. After coronary artery occlusion, all animals exhibited cardiac arrhythmias, which occurred as VPB, VT, and VF. In some animals, atrioventricular (A-V) block also occurred. On subsequent reperfusion, arrhythmia severity was much less marked in those rats given ISL (Table 2). VPB developed in 100% of vehicles versus 62.5%, 42.8%, and 25% (P<0.05) of ISL 5, 10, and 20 mg/kg treated animals, respectively. The incidences of VF that occurred in 83% of the vehicle group were decreased to 0 in the ISL 20 mg/kg group. Also, ISL markedly shortened the duration of ventricular arrhythmias.
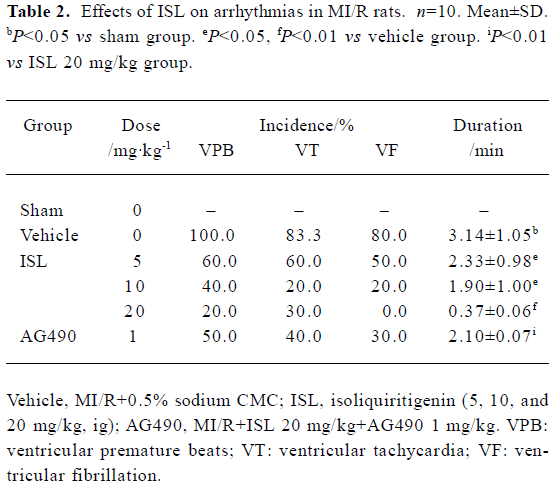
Full table
Effects of ISL on plasma LDH and CPK activities The biochemical indicators of myocardial damage were evaluated in the MI/R period. When plasma LDH and CPK activities in rats treated with ISL, the results shown in Table 3 were obtained. Compared to the vehicle group, pretreatment of rats with ISL (5, 10, and 20 mg/kg, ig) markedly decreased the LDH and CPK activities by 22%, 34%, 38% and 35%, 52%, 58%. The decreases of LDH and CPK releases partly disappeared with the inhibition of AG490.
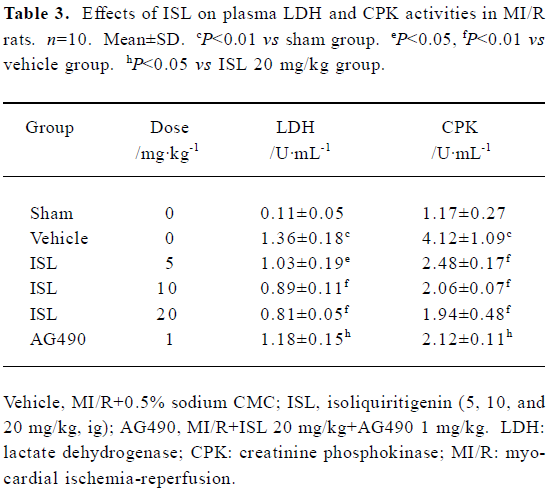
Full table
Effects of ISL on MT-1, MT-2 mRNA and protein expressions This study investigated the effect of ISL on the regulation of MT expressions in MI/R rat model. RT-PCR demonstrated specific amplifications of MT, COX-2, and iNOS cDNA. MT-1 and MT-2 mRNA were upregulated by ISL in a dose-dependent manner. There were no significant differences of COX-2 or iNOS mRNA levels among ISL-treated groups when compared to the vehicle group (Figure 1). With the dose of ISL increased, the protein expressions of MT-1, MT-2 were obviously increased. This result indicated that MT expressions were upregulated by ISL in myocardium through transcriptional activation. In contrast, the increased MT expression in the ISL 20 mg/kg group was blocked by tyrphostin AG490.
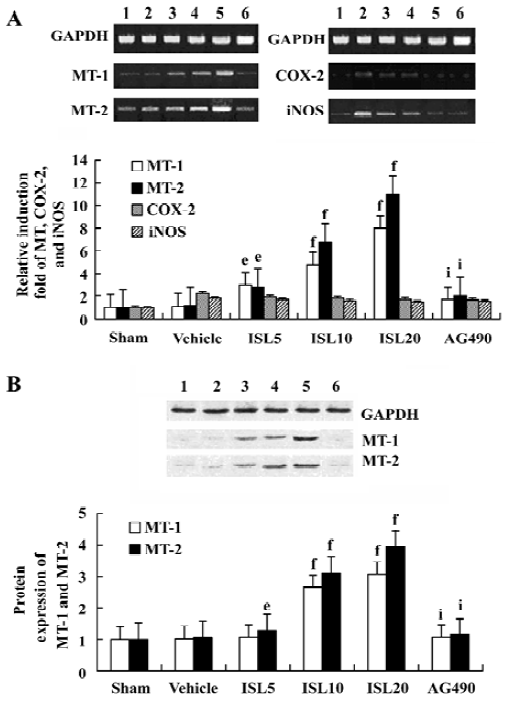
Effects of ISL on JAK /STAT phosphorylations To determine the underlying molecular mechanism of the JAK 2 inhibitor-induced decrease of MT expression, the phosphorylation of JAK 2, STAT 1, STAT 3, ERKs, and Akt were examined by Western blot analysis. The results suggested that pretreatment with AG490 30 min before the coronary artery ligation combined with ISL 20 mg/kg significantly inhibited the phosphorylation of STAT 3. STAT 1 was weakly phosphorylated in MI/R rats, however, it was not obviously affected by AG490 treatment (Figure 2). Other signal transducing molecules downstream of gp130, such as ERK and Akt were also analysed with Western blotting by using phosphor-specific antibodies. As shown in Figure 2, neither Akt nor ERK was activated in MI/R rats of ISL-treated groups, indicating that JAK 2/STAT 3 may be exclusively activated.
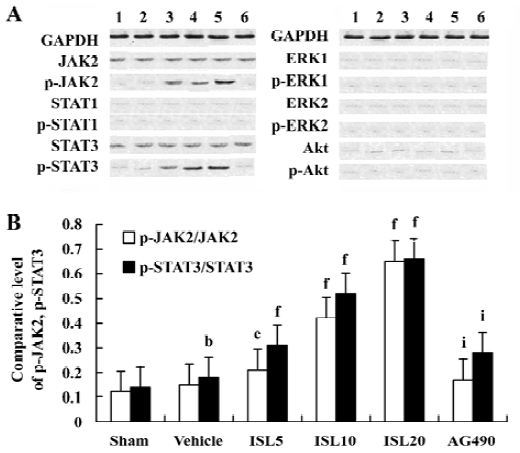
Discussion
In the present study, we demonstrated that ISL induced the upregulation of MT expression. As expected, ISL also resulted in cardioprotection as evidenced by attenuated ventricular arrhythmias, reduced infarct size, and decreased the releases of LDH and CPK. The ISL treated MI/R rats revealed increased phosphorylations of JAK2/STAT3 but not of ERKs and Akt pathway. Inhibition of JAK2 kinase with tyrphostin, AG490 resulted in the inhibition of STAT3 phosphorylation, decreased the expressions of MT-1 and MT-2, and abrogated the cardioprotection effects of ISL.
Myocardial reperfusion through thrombolysis, percutaneous transluminal coronary angioplasty, or coronary artery bypass grafting is standard treatment in acute myocardial infarction. However, these therapies initiate a second phase of myocardial injury either by acceleration of detrimental processes initiated during ischemia or by inducing additional pathological processes following reperfusion[19,20]. Our results indicated that in MI/R model group, serious myocardial infarction and ventricular arrhythmias were observed. Besides this, the activities of LDH and CPK in the plasma were much higher than those in the sham group.
Increasing clinical and experimental data have provided evidence that ischemic cell injury is mediated by ROS[21]. The role of oxygen free radicals in the pathophysiology of ischemia-reperfusion injury is supported by the increased formation of lipid peroxides and other toxic products following such as injury[22]. The interaction of ROS with cell membrane lipids and essential proteins contributes to myocardial cell damage, leading to inflammatory reactions, irreversible tissue injury, and consequently, to impaired cardiac function[23].
MT, a low molecular protein rich in L-cysteine have been shown to be associated with resistance to oxidative stress in cardiac protection against ischemia-reperfusion damage[24]. In this study, we found that both the mRNA and protein expressions of MT were upregulated by ISL. These results are in accordance with previous studies on the antioxidative role of ISL.
Originally described as the regulator for cytokine signaling, JAK/STAT pathway is now recognized as an important membrane-to-nucleus signaling pathway for a variety of stress responses including ischemia, hypoxia, and oxidative stress. In response to ischemic stress, phosphorylation of JAK occurs followed by tyrosine phosphorylation and dimerization of cytosolic STAT monomers. The STAT dimers readily translocate to the nucleus, bind to the promoter regions of the DNA and regulate transcription of genes[25]. The JAK/STAT pathway, especially the JAK2/STAT3-dependent pathway, may have a cytoprotective effect on stretched myocardium in MI/R hearts[4]. Treatment with JAK2 inhibitor reduced the phosphorylation of STAT1, 3 in MI hearts[26]. STAT3 also showed a transducer protective signal against oxidative stress and doxorubicin-induced cardiomyopathy[27]. The current study showed that ISL increased JAK2/STAT3 phosphorylation levels and led to upregulation of MT levels in MI/R rat. These results are consistent with previously published reports that state that specific activation of STAT3 transducers antioxidative signals through MT and confer resistance against ROS stress in the heart.
It has been reported that COX-2 and iNOS are the downstream targets of the JAK/STAT pathway and play an important role in ischemia-reperfusion injury[4,28]. Thus, we examined the mRNA expression of COX-2 and iNOS by RT-PCR and found no significant difference of iNOS and COX-2 mRNA levels in ISL-treated groups when compared to the vehicle group. These findings suggest that COX-2 and iNOS is may not be involved in ISL-induced cardioprotection.
In summary, we demonstrated that ISL, a flavonoid, strongly reduced myocardial infarct size and prevented reperfusion-induced arrhythmias in the model of MI/R rats in vivo. It also appears that activation of JAK2/STAT3 and subsequent upregulation of MT is instrumental, at least in part, in ISL-mediated cardioprotection.
Acknowledgment
We would like to thank the Center for Drug Nonclinical Safety Studies of Wuhan University for technical assistance during this work.
References
- Margit P, Elizabeth R, Gabor M. Effects of bisaramil on coronary occlusion-reperfusion injury and free-radical-induced reactions. Pharmaco Res 1996;33:327-36.
- Lin WT, Yang SC, Chen KT, Huang CC, Lee NY. Protective effects of L-arginine on pulmonary oxidative stress and antioxidant defenses during exhaustive exercise in rats. Acta Pharmacol Sin 2005;26:992-9.
- Mascareno E, Elshafei M, Maulik N, Sato M, Guo Y, Das DK, et al. JAK/STAT signaling is associated with cardic dysfunction during ischemia and reperfusion. Circulation 2001;104:325-9.
- Bolli R, Dawn B, Xuan YT. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc Med 2003;13:72-9.
- Kang YJ, Li Y, Sun X, Sun X. Antiapoptotic effect and inhibition of ischemia-reperfusion-induced myocardial injury in metallo-thionein-overpressing transgenic mice. Am J Pathol 2003;163:1579-86.
- Kang YJ, Dinender K, Timao L, Pawan KS. Metallothionein, oxidative stress and the cardiovascular system. Toxicology 2000;155:17-26.
- Kang YJ, Li G, Saari JT. Metallothionein inhibits ischemia-reperfusion injury in mouse hear. Am J Physiol 1999;276:993-7.
- Oshima Y, Fujio Y, Nakanishi T, Itoh N, Yamamoto Y, Negoro S, et al. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc Res 2005;65:428-35.
- Bhattacharya A, Chatterjee A, Ghosal S, Bhattacharya SK. Antioxidant activity of active tannoid principles of Emblica officinalis (amla). Chin Pharmacol Bull 1999;37:676-80.
- Kang YJ. The antioxidant function of metallothionein in the heart. Am J Pathol 1999;222:263-73.
- Fugh BA. Herbs and dietary supplements in the prevention and treatment of cardiovascular disease. Cardiovasc Res 2000;3:24-32.
- Kanazawa M, Satomi Y, Mizutani Y, Ukimura O, Kawauchi A, Sakai T, et al. Isoliquiritigenin inhibits the growth of prostate cancer. Eur J Pharmacol 2003;43:580-6.
- Tawata M, Aida K, Noguchi T, Ozaki Y, Kume S, Sasaki H, et al. Anti-platelet action of isoliquiritigenin, an aldose reductase inhibitor in licorice. Eur J Pharmacol 1992;212:87-92.
- Kakegawa H, Matsumoto H, Satoh T. Inhibitory effects of some natural products on the activation of hyaluronidase and their anti-allergic actions. Chem Pharm Bull 1992;40:1439-42.
- Qian LP, Zhu SS, Cao JL, Zeng YM. Isoflurane preconditioning protects against ischemia-reperfusion injury partly by attenuating cytochrome c release from subsarcolemmal mitochondria in isolated rat heart. Acta Pharmacol Sin 2005;26:813-20.
- Zhan C, Yang J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion induced focal cerebral ischemia in rats. Pharmacol Res 2006;53:303-9.
- Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J. Estrogen-like activity of glabrene and other constituents isolated from licorice root. J Ethnopharmacol 2001;78:291-8.
- Chen J, Wu W, Tzvete D, Robert W, Joshua LD. Increased metallothionein in light damaged mouse retinas. Free Radic Res 2004;79:287-93.
- Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res 2000;47:446-56.
- Jolly SR, Kane WJ, Bailie MB, Abrams GD, Lucchesi BR. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res 1984;54:277-85.
- Buttke TH, Sandstrom PA. Oxidative stress as mediator of apoptosis. Immunol Today 1994;15:7-10.
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword. Circ Res 1985;76:1713-9.
- Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation—the two keystones of ischemia/reperfusion injury. Int J Cardiol 2002;86:41-59.
- Shlafer M, Kane PF, Wiggins VY, Kirsh MM. Possible role for cytotoxic oxygen metabolites in the pathogenesis of cardiac ischemic injury. Circulation 1982;66:185-92.
- Ihle JN. STATs: Signal transducers and activators of transcription. Cell 1996;84:331-4.
- Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, et al. Activation of JAK/STAT pathway transduces cytopro-tective signal in rat acute myocardial infarction. Cardiovasc Res 2000;47:797-805.
- Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, Das DK, et al. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. J Mol Cell Cardiol 2001;33:1893-6.
- Ding HL, Zhu HF, Dong JW, Zhu WZ, Yang WW, Yang HT, et al. Inducible nitric oxide synthase contributes to intermittent hypoxia against ischemia/reperfusion injury. Acta Pharmacol Sin 2005;26:315-22.
