Agmatine increases proliferation of cultured hippocampal progenitor cells and hippocampal neurogenesis in chronically stressed mice1
Introduction
Agmatine has long been known as a constituent of bacteria, plants and a range of invertebrates, and has been considered as a precursor of putrescine. In the mammalian brain, agmatine is an endogenous neurotransmitter and/or neuromodulator[1]. Exogenously administered to rodents, agmatine decreases hyperalgesia accompanying inflamma-tion. It normalizes mechanical hypersensitivity (allodynia/hyperalgesia) induced by chemical or mechanical nerve injury and reduces autotomous-like behavior and the lesion area after excitotoxic spinal cord injury[2]. It is quite clear that agmatine may have an antidepressant effect in animal models, which is associated with its blockade of the N-methyl-D-aspartate (NMDA) receptors[3,4]. It has really been demonstrated that peripheral administration of agmatine can cross the blood-brain barrier[5].
In fact, in the whole-cell patch clamp in cultured hippocampal neurons, extracellularly applied agmatine produces a voltage- and concentration-dependent blockade of the NMDA current, but not α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) or kainate currents[6]. Our previous studies showed that agmatine inhibited NMDA-induced neuronal damage and Ca2+ accumulation in PC12 cells[4], the amine also has been shown to inhibit all isoforms of nitric oxide synthase (NOS)[2]. It has been shown that NMDA blockers and NOS inhibitors produce antidepressant effects, indicating that the antidepressant effect of agmatine may be NMDA receptor associated.
Chronic unpredictable stress (and also mild stress) models have been widely used for the evaluation of antidepressants for several decades[7–9]. It has been well reported that both physical and psychosocial stress paradigms, as well as some animal models of depression, decrease adult neuro-genesis in the hippocampus, while chronic (but not acute) treatment with different types of antidepressants reverses the stress-induced decrease of the hippocampal neurogene-sis[10–13]. In 2003, Santarelli et al first found that the hippocampal neurogenesis plays an important role in the behavioral effects of antidepressants[14].
Neurogenesis has been documented in the adult brain of a number of different animals including birds and rodents. In the hippocampus, progenitor cells are located in the dentate gyrus where they divide and give rise to new neurons. Stress-related psychotic diseases, such as depression and post-traumatic stress disorder, may be closely associated with the downregulation of hippocampal neurogenesis[15,16]. Based on current reports in this area and our previous studies, upregulation of the hippocampal neurogenesis in chronically stressed animals is one of the common action mechanisms for antidepressants[10,17]. Given that NMDA antagonists upregulated the hippocampal neurogenesis[18], it is of interest to know whether upregulation of the adult hippocampal neurogenesis is one of the mechanisms involved in the antidepressant action of agmatine. To address this issue, we determined the effects of agmatine, a novel neurotransmitter and NMDA receptor blocker, on the proliferation of the cultured hippocampal progenitor cells in vitro and neurogenesis in the dentate gyrus of hippocampus using the bromodeoxyuridine (BrdU) label technique in chronically stressed mice.
Materials and methods
Animals Male mice (18±2 g) of the Kun-ming strain were provided by the Animal Center of Beijing Institute of Pharmacology and Toxicology, Beijing (Beijing, China). All experiments were carried out according to the NIH Guide for the Care and Use of Laboratory Animals (revised 1996).
Drugs and reagents Agmatine sulfate (a white powder with >99% purity) and imipramine were purchased from Sigma (St Louis, MO, USA); DMEM/F12 (1:1) medium was purchased from Hyclone (Longan, UT, USA). B27, N2 supplements were purchased from Gibco BRL(Gaithersburg, MD, USA); Epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) were purchased from PeproTech Inc.(Rocky Hill, NJ, USA). BrdU, mouse BrdU monoclonal antibody and the BrdU immunohistochemistry detection kit were purchased from Boster Biological Technology Ltd. (Wuhan, China); 3H-thymidine was bought from the Institute of Chinese Atomic Energy of Science (Beijng, China); cell counting kit-8 was bought from Dojindo Laboratories (Kumamoto, Japan) and the goat anti-mouse IgG-FITC, IgG-Cy3 were bought from Sigma (St Louis, MO, USA).
Chronic stress procedure in mice and preparation of brain freezing sections The mice were randomly divided into 4 groups: vehicle control, chronic stress+vehicle, stress+imipramine (10 mg/kg, po) and stress+agmatine (10 mg/kg, po), in which imipramine or agmatine was administered 30 min daily before each stressor. Imipramine and agmatine were dissolved in distilled water with 10 mL/kg administered to the mice. The animals in the normal group and chronic stress group were administered distilled water alone in the same volume as the vehicle control. The chronic stress regimen was based on previous reports[12,13]. Stressors were administered alternately once per d over a 24-d period between 8:30 AM to 10:30 AM. The following 8 stressors were used: cold swimming (10 oC) for 6 min; overhanging for 60 min; foot shock (1 mA, 1-s duration, average 1 shock/min) for 60 min; water deprivation for 24 h; tail pinch (1 cm apart from the end of the tail) for 1 min; food deprivation for 24 h; overnight illumination; and high speed horizontal shaking for 60 min.
For BrdU labeling, the mice were administered BrdU (100 mg/kg, ip, twice per d for 2 d). Twenty-four hours after the last BrdU injection, the mice were killed and perfused with cold saline for 5 min followed by 4% cold paraformaldehyde for 15 min. The brains were post-fixed overnight in cold paraformaldehyde and stored at 4 oC in 30% sucrose. Serial sections (30 µm) of the brains were cut through the hippocampus (-1.3 mm to -3.5 mm from the bregma) on a freezing microtome; the sections were then stored at -20 oC.
In order to find out whether the new born cells could differentiate into new neurons, four mice in the agmatine group continued to be fed normally without any drug treatment for another 4 weeks after the last BrdU injection. The mice were killed and perfused and serial sections (30 µm) of the brains were cut through the hippocampus (-1.3 mm to -3.5 mm from the bregma) on a freezing microtome. The immunofluorescence determinations were processed as follows.
Open-field behavior detection Twenty-four hours after the last stressor and drug treatment, the mice were placed individually in an open field box (35 cm×30 cm×22 cm) fitted with a black rubber floor. The open-field activity of the mice in 10 min was displayed and recorded automatically by using a Videomex-V image analytic system (Columbus Co, Ohio, USA). The parameters observed included the distance travelled, ambulation time and average speed.
Immunohistochemistry determination The operating procedure was mainly performed according to the specifications of a BrdU immunohistochemistry kit with some modification. Briefly, after DNA denaturation and several PBS rinses, the sections were incubated for 30 min in 2 mol/L HCl and then 10 min in 0.1 mol/L borate sodium (pH 8.5). The sections were washed with PBS followed by incubation with methanol containing 0.5% H2O2 for 30 min to eliminate endogenous peroxidases. After being blocked by normal goat serum for 60 min at 37 oC, the sections were incubated with anti-mouse BrdU (1:400) overnight at 4 oC and the secondary antibody (biotinylated goat anti-mouse) for 1 h, which was followed by amplification with a streptavidin-biotin complex. The sections were then visualized with 3,3’-diaminobenzidine(DAB) for about 45 min. BrdU-positive cells were counted and pictured under a Olympus BX50 microscope (Olympus, Tokyo, Japan).
In this study, every sixth section throughout the hippocampus was processed for BrdU immunohistochemistry. All BrdU-positive cells in the dentate gyrus (including subgra-nule zone and hilus) were counted in each section. At least 5 sections were examined per mouse (total 6 mice per group), and statistical analysis was performed on the average number of BrdU-positive cells per section.
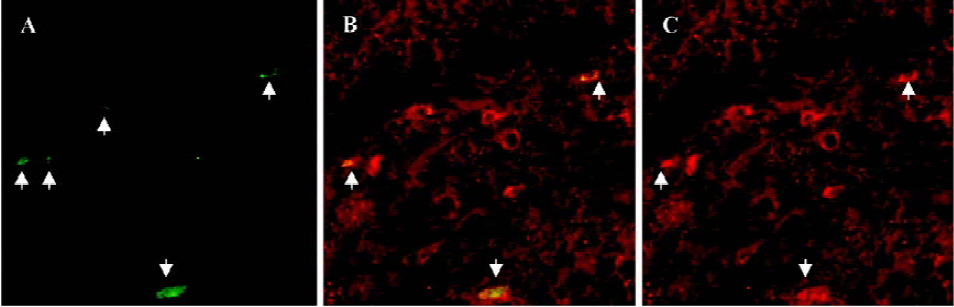
Immunofluorescence labeling To determine whether the BrdU-labeled cells could differentiate into neurons, the sections were doubly labeled for BrdU and neuron specific enolase (NSE; a marker for mature neurons). Sections were first pretreated by incubation in 2×SSC/50% formamide for 2 h at 65 oC, rinsed with PBS, incubated with 2 mol/L HCl for 30 min at 37 oC, and rinsed with 0.1 mol/L borate sodium (pH 8.5) for 10 min. After being blocked for 1 h in PBS (pH 7.5) containing 0.1% Triton X-100 and 3% normal goat serum (PBS-plus), the sections were incubated overnight at 4 oC in an antibody cocktail containing mouse anti-BrdU monoclonal IgG (1:50), mouse anti-NSE monoclonal IgG (1:50) in PBS-plus. The next day, the sections were rinsed with PBS, blocked for 10 min in TBS-plus and incubated with a goat anti-mouse IgG-FITC (1:50) for 1 h at 37 oC in dark conditions. After PBS rinsing, the sections were incubated with goat anti-mouse IgG-Cy3 (1:50) for 1 h at 37 oC in PBS-plus and were mounted with coverslips on glass slides after rinsing with distilled water. Fluorescence was detected using a Bio-Rad Radiance 2100 confocal system (Bio-Rad Laboratories Inc, Hercules,CA, USA) in conjunction with a Nikon TE300 microscope (Nikon, Tokyo, Japan). The confocal laser microscope was equipped with an argon laser emitting at 488 nm and a helium/neon laser emitting at 543 nm. The images were viewed through a 40×lens (Nikon Plan Fluo) image in a single optical plane. And the different channel images were acquired sequentially.
Hippocampal progenitor cell culture and cell proliferation detection The hippocampus was isolated from the brain of 1-d-old rats. The dissections and culture methods were carried out as described by Zhang et al[19]. Briefly, the dissected hippocampi were digested, spun down, washed and then resuspended in serum-free DMEM/F12 medium supplemented with 2% B27, 1% N2, 20 ng/mL EGF, 20 ng/mL bFGF, 200 kU/L penicillin and 100 mg/L streptomycin. The cell suspension was seeded in a 50 mL culture flask (Costar, Costar Co, Cambridge, MA, USA) at a density of (4-5)×105 cells/mL and cultured in a 5% CO2 incubator at 37 oC. Half of the culture medium was changed every 3 d. Seven days after the cultures, the neurospheres were collected and blown into single cell suspension with Pasteur pipette. The cells were then seeded in 0.1% polylysine-coated 96-well plates at a density of 2×104 cells/mL and were fed in a 5% CO2 incubator at 37 oC. After twenty-four h, the cells were treated with 0.1, 1 or 10 µmol/L agmatine for an additional 3 d.
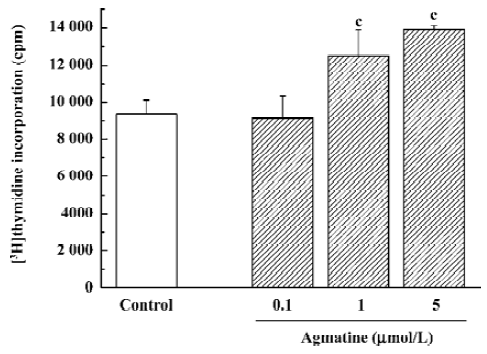
For the 3H-thymidine incorporation assay, 3H-thymidine 1 µCi per well was added and the cells were then maintained in the 5% CO2 incubator at 37 oC for 24 h. The cells were then washed several times with cold PBS and cold trichloracetic acid (100 µL/well), respectively, followed by the addition of NaOH at 0.2 mol/L (100 µL/well) with ultrasonication for 15 min. Scintillation solution was added and the radioactivity was counted in a liquid scintillation β counter (Columbus Instruments, Columbus, OH, USA).
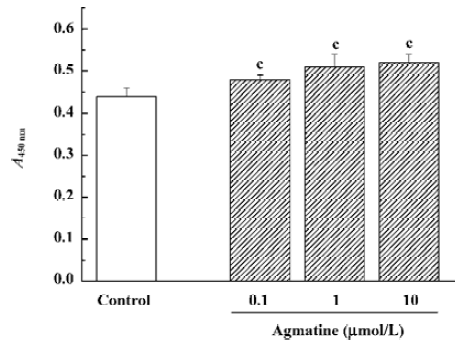
The cell counting kit-8 colorimetric assay was performed following the instructions of the kit. The water-soluble tetrazolium salt-8 (WST-8) reagent (10 µL/well) was added after agmatine treatment. Four h later, absorbance at 450 nm (A450nm values) was detected using a VERSAmax spectrophotometer (Molecular Devices, Union City, CA, USA).
Statistical analysis Values were shown as mean±SD. t-test with grouped designs (the designs were processed 3 times) was used for the behavioral and immunohistochemistry data, while one-way ANOVA and Dunnett’s t-test were used for the cultured cell proliferation data.
Results
Effect of agmatine on the open-field behavior of chronically stressed mice With the onset of chronic stress, the locomotor activity of the stressed mice in the open field significantly decreased, while chronic coadministration of 10 mg/kg imipramine or 10 mg/kg agmatine increased the open-field behavior as shown in Table 1, further indicating that agmatine had an antidepressant effect in the chronically stressed model of mice.
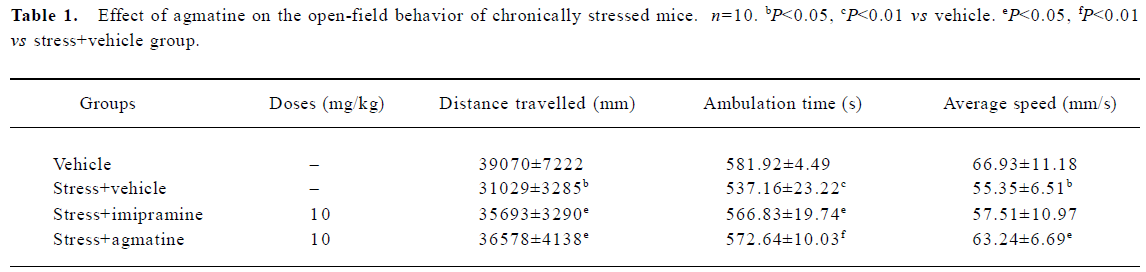
Full table
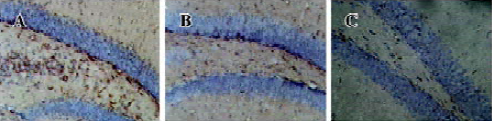
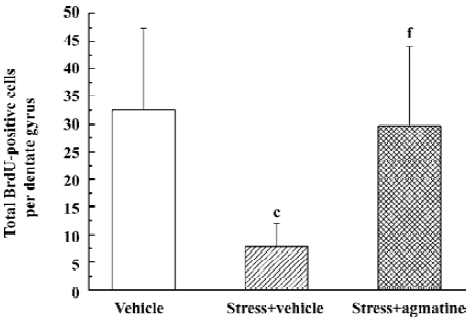
Effect of agmatine on the number of BrdU-labeled cells in the dentate gyrus of chronically stressed mice BrdU, which incorporates into DNA in S-phase cells, is a label for proliferating cells. In chronically stressed mice, BrdU-positive cells (shown as brown granules) in the dentate gyrus of the hippocampus decreased compared to the vehicle. This was blocked by chronic treatment with agmatine 10 mg/kg (po), which significantly increased the number of BrdU-positive cells in the hippocampal dentate gyrus (Figures 1,2). Four weeks after the last injection of BrdU, some of the BrdU-positive cells in the dentate gyrus coexpressed the mature neuron marker NSE, indicating that some new born cells differentiated into neurons (Figure 3).
Effect of agmatine on the proliferation of progenitor cells from the neonatal rat hippocampus In our previous studies, hippocampal progenitor cells from neonatal rats were identified; they are nestin and BrdU positive and capable of proliferation in the presence of EGF and bFGF[19]. The cell counting kit-8 is a colorimetric assay for the number of viable cells for the determination of cell proliferation, in which the WST-8 is reduced by dehydrogenases in cells to give a yellow-colored formazan. The amount of the formazan dye generated by dehydrogenase activity in cells is directly proportional to the number of living cells. Using this colorimetric assay, it was found that treatment with agmatine 0.1–10 µmol/L for 3 d significantly increased the proliferation of cultured hippocampal progenitor cells in a dose-dependent manner (Figure 4). Thymidine is one of the required materials for DNA synthesis, so 3H-Thymidine can incorporate into the newly synthesized DNA. Therefore, the radioactivity of 3H-Thymidine in cells can indicate cell proliferation. Using this assay, agmatine treatment of 1 or 5 µmol/L for 3 d significantly increased radioactivity by 33.7% or 48.6%, respectively, further indicating that agmatine promotes the proliferation of hippocampal progenitor cells (Figure 5).
Discussion
It has been reported that chronic unpredictable stress (stronger than mild stress) paradigms can be well used for antidepressant evaluation through the detection of open-field behavior[7,8]. Our previous studies found that agmatine had no effect on locomotor activity, at least in the dose range for its antidepressant actions[4]. In this study, imipramine or agmatine reversed the chronic stress-induced decrease of open-field behavior, further indicating a significant antidepressant action of agmatine. In our previous study, it was also found that the same stress regimen downregulated the hippocampal neurogenesis in mice, which was blocked by the chronic treatment of fluoxetine or desipramine[12]. Agmatine had the same effect in this study. Interestingly, we also found that coadministration of 5-fluorouracil (15 mg/kg; a toxin for proliferating cells without impairing overall health at this dose), abolished the chronic behavioral efficacy of agmatine in the novelty suppressed feeding test and sucrose consumption test in mice (unpublished data), further suggesting that the hippocampal neurogenesis is involved in the chronic antidepressant and anxiolytic efficacy of agmatine.
Accumulating data suggest that antidepressant and anxiolytics elicit some of their effects through NMDA receptor mechanisms[20]. First, antidepressants inhibit the function of NMDA receptors in the brain. Second, NMDA receptor antagonists, such as dizocilpine (MK801) or (±)-2-Amino-7-phosphonopentanoic acid (AP-7), produce antidepressant- and anxiolytic-like effects in rodents[20]. Finally, our previous studies support the view that inhibition of the NMDA receptor function and neuroprotection may be one of the common actions of antidepressants[21,22]. Recently, we also found that agmatine exerts anxiolytic action[23]. Furthermore, agmatine can protect PC12 cells[4] and cultured rat hippocampal neurons (unpublished data) against NMDA excitotoxi-city and inhibits Ca2+ overload and NOS activity in cells. All these results indicate that inhibition of the NMDA-Ca2+-NOS pathway, at least partially, can underlie the antidepressant action of agmatine.
In the hippocampus, progenitor cells are located in the dentate gyrus where they proliferate and differentiate into new neurons. A recent analysis reports that there are approximately 9000 new cells per d in the adult rodent hippo-campus. Approximately 70% of these cells differentiate and express cellular markers of neurons. As previously reported, this proportion is not influenced by antidepressant treatment[24]. It is also estimated that new granular neurons represent about 60% of the afferents from the entorhinal cortex and 30% of CA3 pyramidal cells receiving efferent projections from granule cells[16]. In the present study, it was found for the first time that agmatine increases the proliferation of hippocampal progenitor cells in vitro and reverses stress-induced decreases of new born cells (BrdU positive) in dentate gyrus in vivo, which is consistent with the effects of classical antidepressants in our previous study[12,19]. Also, the surviving new born cells coexpressed neuronal marker NSE 4 weeks later. These results suggest that agmatine increases adult neurogenesis of the hippocampus in stressed mice.
It has been well reported that NMDA antagonists upregu-late the hippocampal neurogenesis[18], suggesting that agmatine-induced hippocampal neurogenesis upregulation may be also associated with its NMDA receptor blockade. In summary, agmatine-induced hippocampal neurogenesis upregulation and neuroprotection, both of which are related to its blockade on NMDA receptors, may be underlying its antidepressant and anxiolytic actions.
References
- Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol Sci 2000;21:187-93.
- Fairbanks CA, Schreiber KL, Brewer KL, Yu CG, Stone LS, Kitto KF, et al. Agmatine reverse pain induced by inflammation, neuropathy, and spinal cord injury. Proc Natl Acad Sci USA 2000;97:10584-9.
- Zomkowski AD, Hammes L, Lin J, Calixto JB, Santos AR, Rodrigues AL. Agmatine produces antidepressant-like effects in two models of depression in mice. Neuroreport 2002;13:387-91.
- Li YF, Gong ZH, Cao JB, Wang HL, Luo ZP, Li J. Antidepressant-like effect of agmatine and its possible mechanism. Eur J Pharmacol 2003;469:81-8.
- Piletz JE, May PJ, Wang G, Zhu H. Agmatine crosses the blood-brain barrier. Ann N Y Acad Sci 2003;1009:64-74.
- Yang XC, Reis DJ. Agmatine selective blocks the N-Methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Ther 1999;288:544-9.
- Roth KA, Katz RJ. Further studies on a novel animal model of depression: therapeutic effects of a tricyclic antidepressant. Neurosci Biobehav Rev 1981;5:253-8.
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev 1981;5:247-51.
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev 1992;16:525-34.
- D’Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord 2002;4:183-94.
- Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. Rev Psychiatry Neurosci 2004;29:196-205.
- Li YF, Liu YQ, Zhang YZ, Yuan L, Luo ZP. Effect of antidepressants on the hippocampal neurogenesis in chronically stressed mice. Chin J Pharmacol Bull 2004;20:385-8.
- Li YF, Zhang YZ, Liu YQ, Wang HL, Yuan L, Luo ZP. Moclo-bemide up-regulates proliferation of hippocampal progenitor cells in chronically stressed mice. Acta Pharmacol Sin 2004;25:1408-12.
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301:805-9.
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry 1999;46:1472-9.
- Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressants treatment. Neuropsychopharmacology 2001;25:836-44.
- Li YF, Luo ZP. Depression: neuron lesion and neurogenesis down-regulation. Acta Pharm Sin 2004;39:949-53.
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging 2003;24:273-84.
- Zhang LM, Li YF, Gong ZH. Culture of hippocampal progenitor cells from neonatal rats and the effect of antidepressants on the cell proliferation. Chin J Clin Rehab 2005;13:53-6.
- Petrie RXA, Reid IC, Stewart CA. The N-methyl-D-aspartate receptor, synaptic plasticity, and depressive disorder: a critical review. Pharmacol Ther 2000;87:11-25.
- Li YF, Zhang YZ, Liu YQ, Wang HL, Cao JB, Guan TT, Inhibition of -methyl--aspartate receptor function appears to be one of the common actions for antidepressants. J Psycho-pharmacol 2006; [Cited in 9 January 2006] Available from URL: http://jop.sagepub.com/cgi/rapidpdf/0269881106059692v1.pdf
- Li YF, Liu YQ, Luo ZP. Cytoprotective effect is one of the common action pathways for antidepressants. Acta Pharmacol Sin 2003;24:996-1000.
- Gong ZH, Zhao N, Yang HJ, Su RB, Luo ZP, Li J. Anxiolytic effect of agmatine on anxiety animal models. Pharm J Chin PLA 2005;21:163-7.
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increase neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104-10.
