Effect of leukemia inhibitory factor on embryonic stem cell differentiation: implications for supporting neuronal differentiation1
Introduction
Leukemia inhibitory factor (LIF) is capable of maintaining embryonic stem (ES) cells in a pluripotent state through promoting self-renewal or suppressing stem cell differentiation[1–5]. It has become a standard protocol to use LIF to maintain murine ES cell pluripotency, whereas withdrawal of LIF allows ES cells to undergo cell differentiation[1–7]. Upon withdrawal of both LIF and feeder cells, ES cells are able to differentiate spontaneously into various cell types in three primitive layers[8–11].
As a pleiotropic factor, LIF has been proven to have a wide array of actions during the process of neural progenitor cell differentiation into neuronal and glial cells[12–19]. In the central nervous system, the LIF signaling pathway synergistically functions with other signaling pathways to inhibit the differentiation of neural stem cells along a glial lineage[12,13]. However, LIF downstream factor STAT3, in combination with other factors, selectively promotes fetal neural stem cell differentiation toward a glial fate[14–16]. During neural crest cell differentiation, LIF enhances the survival of sensory neurons and stimulates their formation from neural crest progenitor cells[17–19]. When ES cells are cultured in a chemically defined serum-free medium designed for neuronal cell culture, LIF alone is insufficient to sustain ES cell renewal[5]. Furthermore, when cultivated in low-density and serum-free conditions, ES cells with neural progenitor properties can be identified. It seems that LIF is critically required under these conditions for ES cells to undergo neuronal colony formation[20]. These observations form the basis for the hypothesis that LIF might have a role in mediating the neural development of ES cells.
ES cell transition from pluripotency towards a specific cell type involves the actions of multiple cytokines. Removing LIF from culture medium seems to allow ES cells to differentiate into nearly all kinds of cell types, but it is unclear if LIF plays a role during ES cell differentiation towards a specific cell type[8–11,21]. In previous studies, either in retinoic acid (RA)-induced or non-induced ES cell differentiation, LIF was removed from culture medium. However, whether LIF plays a role in inhibiting neural differentiation during this process remains unknown[22–24,27,28].
To better understand whether LIF potentially exerts a role during the conversion of ES cells to a neuronal fate, embryoid bodies (EB) were obtained from ES cells and plated for further culture in a serum-containing medium either with (LIF+) or without LIF (LIF–), and then analyzed for cell differentiation.
Materials and methods
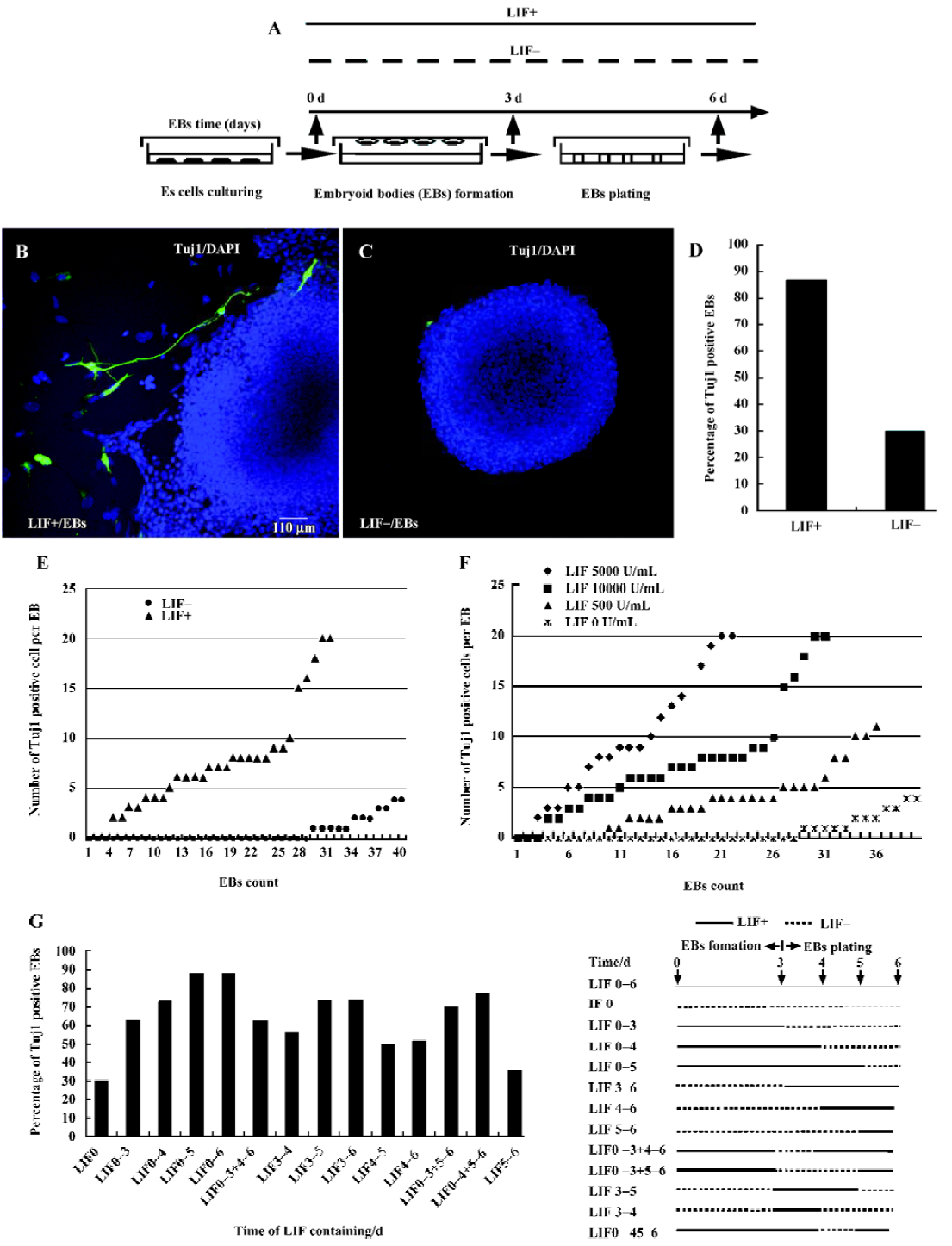
ES cell culture and embryoid body formation The mouse ES cell line D3 was obtained from the ATCC (American Type Culture Collection) organization, and was maintained in an undifferentiated state with feeder cells in the presence of LIF (ESGRO; Chemicon USA)[1–5]. The standard culture medium used in this work for maintaining ES cells contained the following components: high glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) supplemented with 15% fetal calf serum (Hyclone USA or Sijiqing China), 0.1 mmol/L β-mercaptoethanol (Gibco USA), 1 mmol/L sodium pyruvate (Gibco USA), 1% non-essential amino acids (Gibco, USA), 2 mmol/L glutamine (Gibco, USA), 0.1 mg/mL penicillin-streptomycin (Gibco, USA). LIF was added into the culture medium at a final concentration of 1000 U/mL before use. For cell differentiation induction, ES cells were dispersed into a single cell suspension with 0.25% trypsin (Gibco, USA). Hanging drops, each of which contained approximately 1000 cells in a 20 µL volume of culture medium, were maintained for 3 d on the lids of dishes filled with phosphate-buffered saline (PBS)[22–24]. EB that formed in the hanging drops were then plated and cultured on uncoated Petri dishes or cover-slips (Fisher) for 3 d. Prior to hanging drop culture, the expression of pluripotent marker genes Oct3/4, alkaline phosphatase (AP) and SSEA-1 was examined to ensure that the ES cells were indeed in an undifferentiated state (data not shown)[1–2]. The optimal procedure, shown in Figure 1A, includes suspension of ES cells for 3 d to form an EB (3 d EB), followed by plating EB cells for an additional 3 d (6 d EB). To assess the potential role of LIF during EB differentiation, the same concentration of LIF as that used in ES cell-maintaining medium was either included (LIF+, 1000 U/mL) or excluded (LIF–) in culture medium during the suspension and plating processes.
EB treatment For RA induction, 6 d EB were treated with 10-6–10-5 mol/L all-trans RA (Sigma) for an additional 3 d and then transferred to fresh medium without LIF[22–24]. To block the LIF downstream signaling pathway, MEK1/2 specific inhibitor PD98059 (Calbiochem, USA) was dissolved into 100% EtOH and was added into culture medium with LIF at a final concentration of 2 µmol/L during either EB formation or EB plating; JAK/STAT3 specific inhibitor cucurbitacin I (Calbiochem, USA) was also dissolved into 100% EtOH and used to inhibit JAK/STAT3 function at a concentration of 300 nmol/L. The 6 d EB were fixed and analyzed with immunostaining.
Immunocytochemical assays SSEA-1 was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA). The monoclonal antibody Tuj1, a neuron-specific marker, was obtained from Sigma (St Louis, MO, USA), and the antibodies GFAP (Dako), a glial marker, and the antibodies against GATA-4, an extraembryonic endoderm marker, and brachyury, a mesoderm marker, were from Santa Cruz (Santa Cruz, CA, USA). For immunocytochemical assays, cells were fixed with 4% paraformaldehyde (PFA) in PBS for 10 min, permeabilized with 0.1% Triton X-100/PBS for 1 min, washed with PBS 3 times, and blocked with 5% normal goat serum in PBS for 1.5 h. Samples were then incubated with primary antibodies in 10% NGS (normal goat serum)-PBS at 4°C for 12 h. After extensive washing with PBS, samples were incubated with secondary antibodies in 10% NGS-PBS for 1 h, washed again with PBS and then mounted in mowiol (Sigma USA). Specimens were analyzed under a confocal microscope (Zeiss L510 or Leica confocal microscope).
Cell proliferation and apoptosis assays Bromode-oxyuridine (BrdU; Sigma USA) was used to label proliferating cells in 5 d, 8 d (RA treated for 2 d) and 10 d (RA treated for 3 d and cultured for an additional 1 d) EB. BrdU was added into the culture medium at a concentration of 10 nmol/L for 12–16 h. Cells were washed with PBS for 10 min and fixed with 4% PFA for 15–20 min, then washed with PBS again for 15 min. Samples were then incubated in 2 N HCl for 5–0 min, followed by PBS washing for 15 min. EB were rinsed with Na2B4O7 (0.1 mol/L) for 5–10 min to neutralize the HCl. Samples were then immunostained with BrdU antibodies and analyzed with a confocal microscope. TUNEL (Promega, USA) staining was performed according to the manufacturer’s instructions. Specimens were mounted by mowiol and analyzed with a confocal microscope.
Statistics of apoptosis and proliferation EB were scanned into stacks of images (5 µm/images) with a confocal laser microscope. The middle image for each EB was used, and the number of TUNEL- or BrdU-labeled cells in the image was automatically counted by IPP (Image-Pro-Plus) software using the same parameters. The area of EB on each image was enclosed with the lasso or marquee tool of Photoshop software. We read out the pixel value of the enclosed area in a histogram. The number of BrdU- or TUNEL-labeled cells divided by the pixel value is the relative density of BrdU- or TUNEL-labeled cells per image, respectively. Thirty EB were counted in each case. Student’s t-test was used on these data.
Results
Production of neuronal cells from ES cells was enhanced by LIF under various differentiation conditions ES cells in hanging culture formed spheroid aggregates, known as EB, that can differentiate into a large variety of cell types[8,9,21–29]. In previous reports, EB formation and differentiation occurred under LIF-free conditions[22–24]. However, studies regarding neural progenitor cell differentiation and ES cell neuronal fate commitment imply that LIF might play roles in ES cell neuronal transition. To assess this potential function we cultured LIF under various conditions and investigated neuronal differentiation thereafter.
The expression of neuronal specific marker Tuj1 was analyzed by immunostaining primary 6 d EB cultured in either LIF+ or LIF– medium. As shown in Figure 1, numerous Tuj1-positive cells were seen surrounding or within LIF+ 6 d EB (Figure 1B), whereas Tuj1-positive cells were rarely observed in LIF– 6 d EB (Figure 1C). The percentage of the primary EB that contained Tuj1-positive cells in LIF+ medium (27/31) was approximately two times greater than that for EB cultured in LIF– medium (12/40) (Figure 1D). Moreover, the average number of Tuj1-positive cells per EB was only 2 in LIF– medium (Figure 1E), whereas LIF+ EB had an average of 8.5 Tuj1-positive cells per EB, with up to 20 positive cells in some extreme cases (Figure 1E; these results were from 6 replicated experiments). To test whether different batches of sera, different numbers of cell passages, or different cell lines could potentially influence the result, we repeated the whole experiment with different batches of sera, with ES cells with various numbers of passages (8, 12, 15, 20 passages), and with the feeder-free E14.1 ES cell line, and obtained similar results (data not shown)[30,31]. LIF is thus strongly implicated in promoting the differentiation of ES cells to neuronal cells.
To verify whether LIF has dose-dependent or time-window effects on ES cell differentiation to neuronal cells, we cultured EB in media containing different concentrations of LIF (5000 U/mL, 1000 U/mL, 500 U/mL or 0 U/mL). The numbers of Tuj1-positive cells within each EB were counted (the experiment was repeated 3 times). The Tuj1-positive cells per EB when treated with a higher concentration of LIF outnumber those observed when treated with a lower concentra-tion, indicating that the enhancement of neuronal differentiation is indeed dose-dependent within the limited concentration threshold (Figure 1F). To investigate the step at which LIF exerts its action in the course of EB plating, LIF (1000 U/mL) was included in the culture medium at different time points. We found that neuronal differentiation was obviously enhanced by the addition of LIF between d 0 and d 5 of culture, especially between d 3 and d 4 (Figure 1G). Compared with EB cultured in LIF+ medium, irrespective of when LIF was added, the LIF– EB had the least neuronal conversion capacity (Figure 1G).
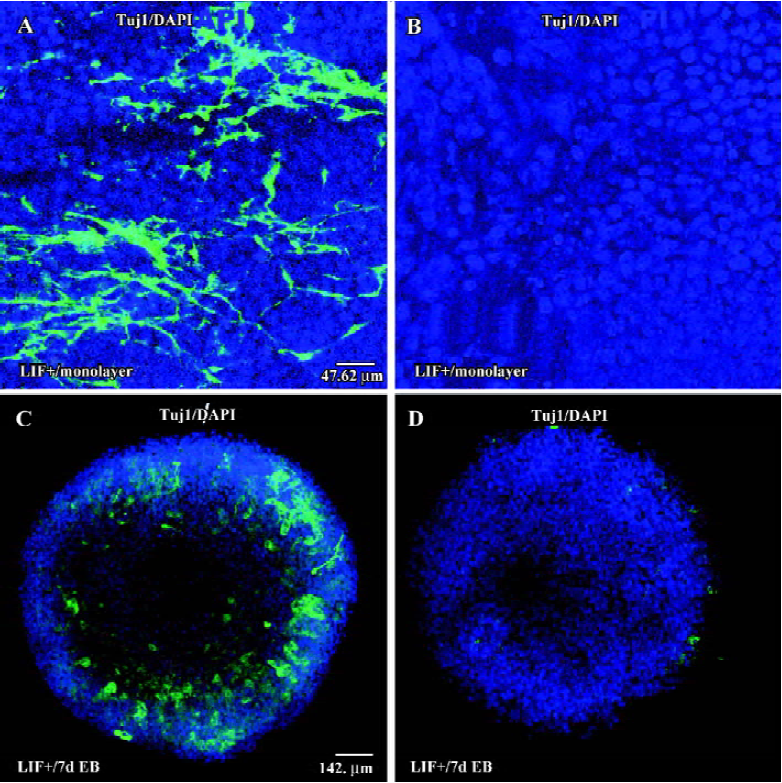
To investigate whether cell-cell interactions could result in the promotion of neuronal conversion in LIF+ medium, we cultured ES cells on cover slides at low density for 6 d to form a monolayer either with or without LIF. After 6 d in culture, some ES cells in LIF+ medium differentiated into Tuj1-positive cells, either in groups or individually (Figure 2A), although the ratio was very low. In contrast, there was no Tuj1-positive cell observed in the culture with LIF-free medium (Figure 2B). In classical RA-induced neuronal differentiation experiments, ES cells are cultured under LIF– conditions, although there is no evidence indicating that LIF inhibits the neuronal differentiation of ES cells[22–24]. To test the potential possibility that LIF also has a positive effect on the RA-induced neuronal differentiation of ES cells, we carried out RA induction assays. We found that LIF did enhance neuronal differentiation in the presence of RA, as evidenced by the fact that LIF+7 d EB (with RA induction for 1 d) generated more Tuj1-positive cells (Figure 2C) than did LIF–7 d EB (Figure 2D). Our results strongly imply that the LIF signaling pathway has a positive role during the neuronal differentiation of ES cells.
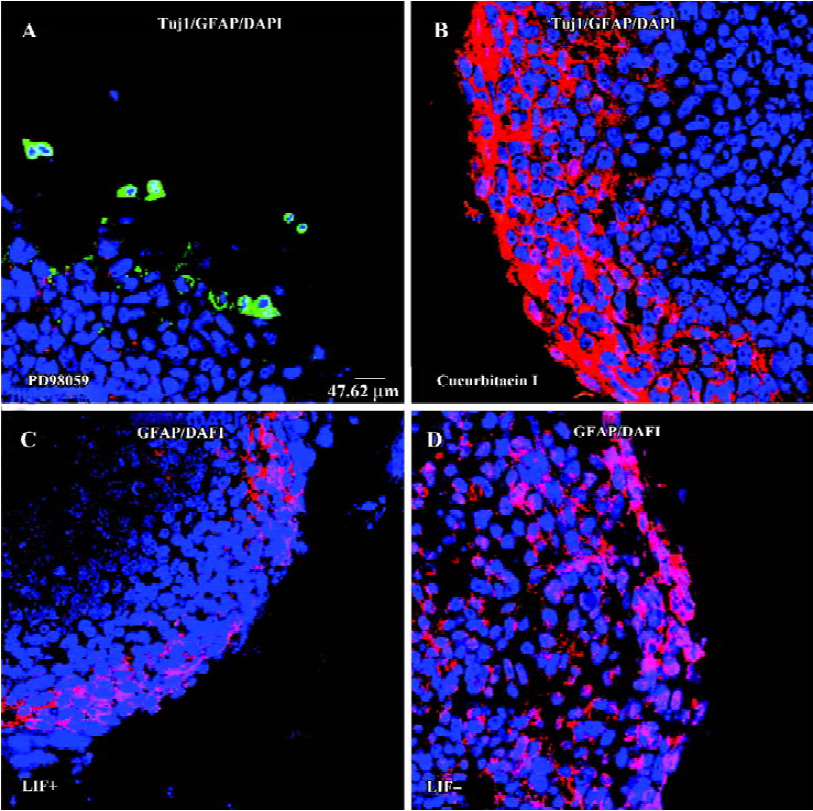
LIF signaling pathway is involved in the differentiation of ES cells to a neural fate LIF plays various roles in different cell types through gp130-mediated JAK/STAT3 and ERK/MEK signaling pathways[32–37]. If LIF does have a positive role in the transition of ES cells to a neuronal fate, this can be validated by inhibition of the LIF-activated signaling pathway. The MEK and STAT3 pathways can be inhibited by different specific chemical inhibitors[37,38]. When the JAK/STAT3 pathway was blocked by the specific inhibitor cucurbitacin I (300 nmol/L)[38] during EB formation or EB plating, neuronal differentiation was largely abolished (Figure 3B). Furthermore, when ES cells were treated with MEK1/2 specific inhibitor PD98059[37] (2 mmol/L) during either EB formation or EB plating, Tuj1-positive cells appeared within or surrounding EB, but these Tuj1-positive cells had a round shape without a long neurite (Figure 3A). Thus, blocking either LIF downstream signaling pathway interfered with LIF-enhanced ES cell neuronal differentiation. However, glial fibrillary acidic protein (GFAP)-positive glial cells are slightly reduced in the presence of LIF (Figure 3C,3D). Inhibition of the STAT3 pathway increased the production of GFAP-positive cells (Figure 3B), whereas there was no GFAP-positive cell observed when the MEK pathway was inhibited (Figure 3A). This result indicates that both LIF-activated signaling pathways are involved in the regulation of glial differentiation from EB.
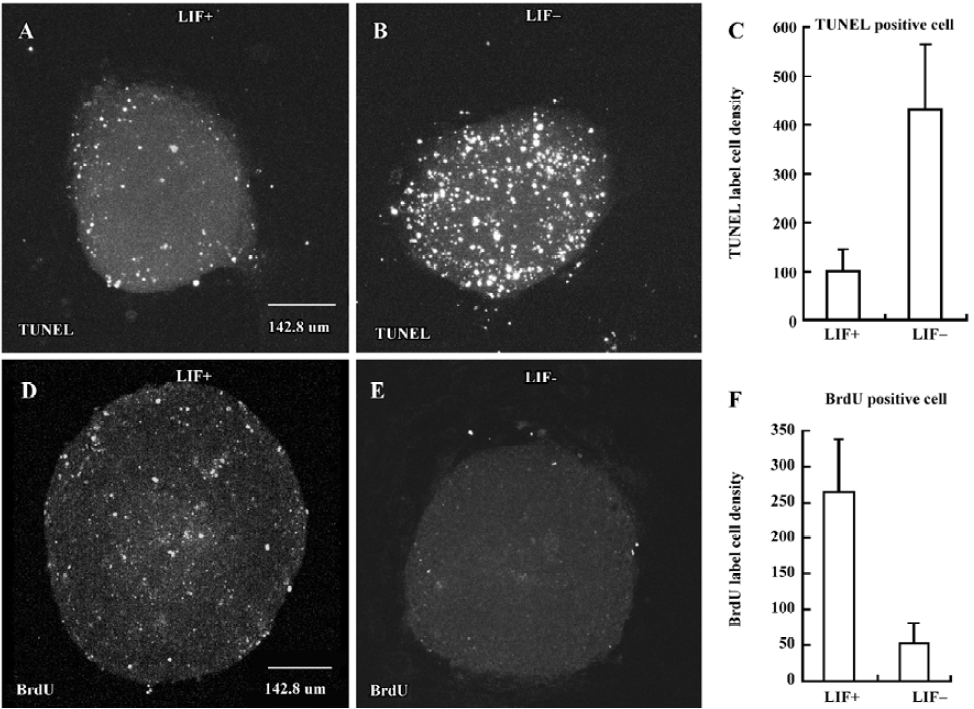
LIF inhibits cell apoptosis and enhances cell proliferation during EB differentiation To examine whether apoptosis and proliferation are also involved in the course of LIF-enhanced ES cell neuronal differentiation, TUNEL and BrdU incorporation assays[1,39] were performed in 5 d either in LIF+ (Figure 4A,4D) or in LIF– medium (Figure 4B,4E), respectively. In the presence of LIF, cell apoptosis was inhibited (Figure 4A–4C) and cell proliferation was enhanced (Figure 4D–4F) during both EB plating and RA treatment. However, LIF did not selectively suppress neuroectodermal precursor apo-ptosis and promote neuroectodermal precursor proliferation. In BrdU and TUNEL assays with nestin staining, we observ-ed that nestin-positive cells exhibited signs of apoptosis and proliferation (data not shown).
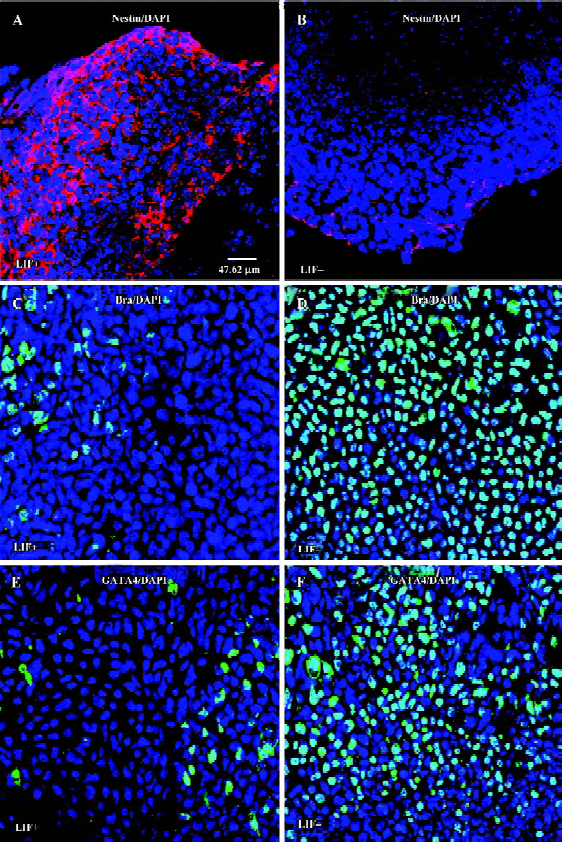
LIF selectively enhances the commitment of neural progenitor from ES cells ES cells are capable of differentiating into various types of cells, whereas the differentiation of cardiomyocytes derived from EB can be hindered by LIF[29]. It remains unclear whether LIF selectively promotes the production of neuroectodermal precursor cells, or if LIF also has a role in mesoderm or extraembryonic endoderm cell lineage commitment. Under LIF+ conditions, a large number of nestin-positive cells appeared within or surrounding EB (Figure 5A), whereas EB cultured without LIF had a small number of nestin-positive cells (Figure 5B), indicating that LIF promotes the production of neural progenitors from ES cells. We also evaluated the expression levels of the mesoderm marker Bra (Brachyury) in 6 d EB cultured either with or without LIF[40]. The number of brachyury-positive cells in 6 d EB with LIF was significantly smaller than that for 6 d EB without LIF (Figure 5B,5C). In addition, it has been reported that ectopic GATA-4 expression is sufficient to induce ES cell differentiation into extraembryonic endoderm[41]. We found that in the absence of LIF the 6 d EB had a larger number of GATA-4-expressing cells surrounding the EB, whereas GATA-4 expression was dramatically reduced in 6 d EB with LIF (Figure 5D,5E). We therefore suggest that LIF potentiates the differentiation of ES cells to a neural fate, and might suppress both the mesoderm and extraembryonic endoderm fates.
Discussion
In this work, we observed that neuronal differentiation of ES cells was enhanced in the presence of LIF. This enhancement cauld be abolished when the JAK/STAT3 pathway was inhibited. Furthermore, inhibition of the MEK signaling pathway impaired the differentiation of ES cells toward a glial lineage. During this process, LIF inhibited cell apoptosis and promoted cell proliferation, and EB differentiation to form both mesodermal and extraembryonic endodermal cells ws inhibited, whereas the production of neural progenitor cells was increased. Thus our observations imply that the LIF signaling pathway is involved in ES cell differentiation into a neuronal cell fate, and provide cues for further investigations of this pathway.
During early vertebrate embryonic development, neural induction has been thought to be a default process. In the default model, the inhibition of the BMP (bone morphogenetic proteins) signaling pathway is necessary for neural cell fate determination during early embryonic development[42–44]. However, it remains unknown whether neural induction requires the participation of other signaling pathways other than simply BMP depletion. The clue that LIF might be involved in ES cell neural differentiation comes from the fact that in low-density and serum-free culture conditions, ES cells with neural progenitor properties were identified[20]. Under these culture conditions, LIF was required for ES cells to undergo neuronal colony formation. In the present report we provided evidence that LIF could enhance the production of neural progenitor cells from ES cells, and support the neuronal differentiation of EB in a dose-dependent manner (Figure 1). Furthermore, in the presence of LIF, the commitment of ES cells to both mesodermal and extraembryonic endodermal fates was suppressed (Figure 5). The LIF pathway also regulates neural differentiation into neuronal and glial fates. Our results not only indicate that LIF signaling is indeed involved in ES cell neural commitment, but also strongly suggest that the LIF pathway has multiple functions in different stages of ES cell differentiation.
A recent study has shown that LIF/Stat3 cooperated with BMP/Smad signaling to maintain ES cells in an undifferentiated state[5]. It seems that the balance between LIF/Stat3 and BMP/Smad signaling is critical for the choice between sustaining pluripotency or lineage commitment of ES cells. Thus the activation of the LIF signaling pathway reported here may account for promoting more ES cells to adapt to a neural cell fate, whereas LIF withdrawal leads to an elevated BMP signaling activity, which further stimulates ES cells to differentiate into mesodermal and endodermal cells. It would be of great interest to further investigate how BMP and LIF signaling pathways cross-talk during ES cell neural commitment.
Multiple lines of evidence indicate that LIF plays various roles in different types of neural stem cell differentiation[12–19]. In the central neuronal system, the LIF signaling pathway synergistically cooperates with other signaling pathways to inhibit neural stem cell differentiation to a glial fate[12,13]. However, LIF downstream factor STAT3, together with BMP downstream effector SMAD1, selectively promotes the glial differentiation of fetal neural stem cells[14–16]. Furthermore, during neural crest cell differentiation, LIF promotes sensory neuron differentiation and survival[17–19]. The LIF pathway requires cooperation with different factors to achieve its function as a pleiotropic cytokine. In our experiments, inhibition of the LIF-activated STAT3 pathway did not interfere with the production of GFAP+ cells, but abolished the differentiation of Tuj1+ cells from ES cells. This result is consistent with the finding that Ngn1 inhibits gliogenesis through inhibiting the activation of STAT transcription factors during neural differentiation[12]. Further-more, blocking the LIF-activated MEK pathway abolished the glial differentiation of ES cells. Our results suggest that LIF signaling might interact with other pathways to exert various effects in the differentiation of different neural cell types. In addition, we found that LIF unselectively inhibited cell apoptosied and supports cell proliferation during ES cell neural differentiation. However, we consider that it would be worthwhile to further investigate the possibility that LIF affects the survival and proliferation of neuronal cells by using more specific cell markers.
LIF is not the only factor that has dual roles in ES cell culture. A recent report indicates that Oct3/4 also has dual roles in murine ES cell culture: sustaining ES cell self-renewal and taking part in ES cell fate determination. The precise level of Oct4 in ES cells controls the commitment of ES cells to undergo 3 different cell fates[1]. Ectopic expression or overexpression of Oct3/4 also appears to enhance neuronal differentiation in SDIA (stromal cell-derived inducing activity)-induced ES cell differentiation[45,46]. However, it is unclear whether LIF signaling interacts with the Oct3/4 signaling pathway in ES cell neuronal differentiation, but this certainly warrants further investigation in the future.
Acknowledgements
We thank Dr Hui-zhen SHENG and Dr Nai-he JING for their technical advice; Dr Peng XIANG for providing the E14.1 cell line; and Prof Yi-ping CHEN, Prof Peter REINACH and Dr Shuang-wei LI for comments on the manuscript. We thank Min-ying LIU for technical assistance.
References
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000;24:372-6.
- Smith AG. Embryo-derived stem cells: of mice and men. Ann Rev Cell Dev Biol 2001;17:455-62.
- Niwa H, Burdon T, Chambers I, Smith AG. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 1998;12:2048-60.
- Smith AG, Nichols J, Robertson M, Rathjen PD. Differentiation inhibiting activity (DIA/LIF) and mouse development. Dev Biol 1992;151:339-51.
- Ying QL, Nichols J, Chambers I, Smith A. BMP Induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003;115:281-92.
- Suda Y, Suzuki M, Ikawa Y, Aizawa S. Mouse embryonic stem cells exhibit indefinite proliferative potential. J Cell Physiol 1987;133:197-201.
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotech 2003;21:183-6.
- Poirier F, Chan CT, Timmons PM, Robertson EJ, Evans MJ, Rigby PW. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development 1991;113:1105-14.
- Adelman CA, Chattopadhyay S, Bieker JJ. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free medium. Development 2002;129:539-49.
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA 1997;94:3801-4.
- Nichols J, Chambers I, Taga T, Smith A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 2001;128:2333-9.
- Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 2001;104:365-76.
- Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci 2001;21:7642-53.
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 1997;78:477-83.
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 1999;284:479-82.
- Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, et al. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci 1999;19:5429-54.
- Murphy M, Reid K, Hilton DJ, Bartlett PF. Generation of sensory neurons is stimulated by leukemia inhibitory factor. Proc Natl Acad Sci USA 1991;88:3498-501.
- Murphy M, Reid K, Ford M, Furness JB, Bartlett PF. FGF2 regulates proliferation of neural crest cells, with subsequent neuronal differentiation regulated by LIF or related factors. Development 1994;120:3519-28.
- Morrow T, Song MR, Ghosh A. Sequential specification of neurons and glia by developmentally regulated extracellular factors. Development 2001;128:3585-94.
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 2001;30:65-78.
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell 2002;110:385-97.
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol 1995;168:342-57.
- Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, et al. Differentiation of embryonic stem cells into adipocytes in vitro. J Cell Sci 1997;110:1279-85.
- Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J Cell Sci 1995;108:3181-8.
- Brustle O, Spiro AC, Karram K, Choudhary K, Okabe S, McKay RD. In vitro-generated neural precursors participate in mammalian brain development. Proc Natl Acad Sci USA 1997;94:14809-14.
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 1999;5:1410-2.
- Drab M, Haller H, Bychkov R, Erdmann B, Lindschau C, Haase H, et al. From totipotent embryonic stem cells to spontaneously contracting smooth muscle cells: a retinoic acid and db-cAMP in vitro differentiation model. FASEB J. 1997;11:905-15.
- Wobus A. M, Rohwedel J, Maltsev V., Hescheler J. In vitro differentiation of embryonic stem cells into cardiomycytes or skeletal muscle cells is specifically modulated by retinoic acid. Roux’s Arch Dev Biol 1994;204:36-45.
- Bader A, Al-Dubai H, Weitzer G. Leukemia inhibitory factor modulates cardiogenesis in embryoid bodies in opposite fashions. Circ Res 2000;86:787-94.
- Miyagi T, Takeno M, Nagafuchi H, Takahashi M, Suzuki N. Flk1+ cells derived from mouse embryonic stem cells reconstitute hematopoiesis in vivo in SCID mice. Exp Hematol 2002;30:1444-53.
- Otani T, Nakamura S, Inoue T, Ijiri Y, Tsuji-Takayama K, Motoda R, et al. Erythroblasts derived in vitro from embryonic stem cells in the presence of erythropoietin do not express the TER-119 antigen. Exp Hematol 2004;32:607-13.
- Ernst M, Oates A, Dunn AR. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J Biol Chem 1996;271:30136-43.
- Boeuf H, Merienne K, Jacquot S, Duval D, Zeniou M, Hauss C, et al. The ribosomal S6 kinases, cAMP-responsive element-binding, and STAT3 proteins are regulated by different leukemia inhibitory factor signaling pathways in mouse embryonic stem cells. J Biol Chem 2001;276:46204-11.
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 1999;18:4261-9.
- Raz R, Lee CK, Cannizzaro LA, d’Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA 1999;96:2846-51.
- Meloche S, Vella FD, Voisin L, Ang SL, Saba-El-Leil M. Erk2 signaling and early embryo stem cell self-renewal. Cell Cycle 2004;3:241-3.
- Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol 1999;210:30-43.
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 2003;63:1270-9.
- Duval D, Reinhardt B, Kedinger C, Boeuf H. Role of suppressors of cytokine signaling (Socs) in leukemia inhibitory factor (LIF)-dependent embryonic stem cell survival. FASEB J 2000;14:1577-84.
- Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 2001;128:3609-21.
- Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, et al. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev 2002;16:784-9.
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development 2004;131:5671-81.
- Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci 2002;3:271-80.
- Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, et al. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev 1995;9:2923-35.
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 2000;28:31-40.
- Shimozaki K, Nakashima K, Niwa H, Taga T. Involvement of Oct3/4 in the enhancement of neuronal differentiation of ES cells in neurogenesis-inducing cultures. Development 2003;130:2505-12.
