Flavonoid-membrane interactions: possible consequences for biological effects of some polyphenolic compounds1
Introduction
Since antiquity plant-derived compounds have been believed to exert a beneficial influence on human health and have been used as medicines. In recent years, various plant components have been isolated, chemically characterized and, in many cases, the mechanisms of their biological action have been established. For many compounds, the spectrum of their biological activity was found to be more broader than was expected on the basis of their most profound biological effects.
The targets for plant-derived drugs have different locations in the cells of the human body. Generally drugs can act on the level of different biological membranes as well as inside the compartments that are limited by these membranes. Even in the latter situation, the drug must still interact with the membrane because the drug must cross it to reach the target. For this reason, the lipophilicity of biologically active compounds is usually one of their most important pharmacological features, and interactions with membranes play an essential role in their biological activity.
The number of different types of substances that are extracted from plants is so large, and their chemical composition is so diverse that it is impossible to summarize the vast literature concerning this field of research. Therefore, in this review I will focus only on one type of plant-derived compounds: flavonoids.
The flavonoids: a short description
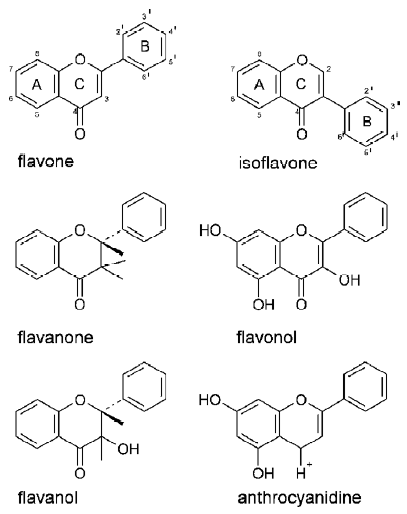
The term flavonoid is used to describe for plant pigments, mostly derived from benzo-γ-pyrone (rings A and C in Figure 1). Flavonoids are abundant in nature and by 1999, almost 6500 different flavonoid structures had been described[1]. Taking into account the chemical nature of the molecule, and the positions of moieties substituting rings A, B, and C, the flavonoids are divided into 14 different groups[2]. Six of the groups are particularly well known and characterized: the flavones, isoflavones, flavanones, flavonols, flavanols (catechins), and anthocyanidines (Figure 1). The chemistry of flavonoids, their classification and natural sources have been reviewed in many papers; 2 recent ones were written by Heim et al[3] and Havsteen[2]. A review dedicated exclusively to the isoprenylated flavonoids was written by Barron and Ibrahim[4]. Depending on the position at which the ring B is attached to the benzo-γ-pyrone core of the molecule, flavonoids (ring B is bound at position 2 of ring C) and isoflavonoids (ring B is bound at position 3 of ring C) are distinguished.
The flavonoids are found ubiquitously in higher plants, and they are present in all parts of plants: starting from the roots and finishing at the flowers and fruits. Because of their occurrence in many edible plants, flavonoids constitute an important component in the majority of people’s daily diets. The common sources of flavonoids are: onions, apples, tomatoes, red wine (quercetin, rutin), grapefruit, black tea (kaempferol), soybeans (genistein, daidzein), parsley, celery (apigenin) and tea (catechins). Apart from flavonoids consumed as food components, some others are extracted from inedible plants and applied as food supplements, for example silibin, which is extracted from milk thistle[5].
The list of flavonoid biological activities is very long[6]. The best-known biological activities of flavonoids include cancer prevention[7,8], hormonal actions[9], cardioprotective actions[10], and inhibitory effects on bone resorption[11]. Despite of the abundance of flavonoids that have beneficial properties, the molecular mechanisms of their actions are very often unknown. The structure-activity relationships of flavonoids are also only partially understood. In the present paper flavonoid-membrane interactions are reviewed, with a special emphasis on the basic molecular mechanisms underlying flavonoid activities.
Antioxidant actions of flavonoids
Perhaps the best known and widely studied biological actions of flavonoids are their antioxidant effects. Their ability to prevent the oxidation of proteins and lipids is also assumed to explain at least some of the positive health effects of flavonoids in humans (cardioprotective and anticancer effects, etc)[12]. It is also important to note that lipid oxidation may delete the domain organization of membranes[13]. Recent research in membrane science has shown the importance of membrane domains (lipid rafts and others) in many cellular processes[14,15]. Because the aim of the present review is to indicate the possible mechanisms of flavonoid biological actions, the effects exerted by crude plant extracts (of unknown composition) will not be reviewed here. Low-density lipoprotein (LDL) oxidation is also excluded from the present review.
The oxidative stability of liposomes containing quercetin and myricetin was compared with those containing α-tocopherol by Gordon and Roedig-Penman[16]. Myricetin appeared to be a stronger antioxidant than α-tocopherol at a concentration of 10-2 mol/mol lipid. Quercetin was a weaker antioxidant than α-tocopherol at this concentration, but its antioxidant activity significantly increased at a concentration of 5×10-2 mol/mol lipid. Another research group studied the antioxidant activity of several isoflavones and their metabolites in liposomal systems[17]. All of the studied compounds showed antioxidant activity; however, their efficacies depended on the system used to initiate lipid peroxidation. It was postulated that the antioxidative potential of isoflavones results from the combination of their radical-scavenging and metal-chelating abilities. The antioxidant potencies of individual isoflavones were also dependent on their chemical structure. The presence of hydroxyl groups in the 5' and 4' positions seemed to be of greatest importance. Isoflavone metabolites, especially equol and its derivatives appeared to be even more efficient antioxidants than their parent compounds. Prior to the research of Arora et al[17] the structural features governing the antioxidant and metal-chelating flavonoid properties were considered by van Acker et al[18]. The presence of the catechol structure in ring B, the 2,3 double bond and the 3-hydroxyl group were thought to be crucial for the antioxidant properties of flavonoids. Furthermore, the 3-hydroxyl group was described as a chelation site.
The idea that the antioxidant properties of flavonoids are not simply and directly related to their lipophilicity was confirmed by the study of Liao and Yin[19]. The antioxidant potency of several flavonoids followed the order catechin > epicatechin>rutin>quercetin>myricetin, both in liposomes and human erythrocyte membranes. This order was negatively correlated with the lipid-water partition coefficients of the studied flavonoids. An interesting finding was that the antioxidant potency of flavonoids was enhanced when they were applied together with other antioxidant compounds (α-tocopherol, β-carotene or vitamin C).
The scavenging activity of 12 flavonoids (4 flavanones, 2 flavones, 5 flavonols, 1 catechin) with respect to peroxyl radicals was measured using an ESR technique by Madsen et al[20]. Flavonols appeared to be the strongest radical scavengers, whereas the scavenging potency of other flavonoids was much less. The results presented in that work confirmed that optimal antioxidative properties are related to the presence of the following structures in flavonoid molecules: catechol (o-dihydroxy) group in ring B, and the 2,3 double bond in conjunction with the 4-carbonyl group as well as the 3- and 5-hydroxyl groups. Thus the hydrophilic/lipophilic balance is of some importance for antioxidant properties of flavonoids.
The free radical scavenging activities of flavonoids and isoflavonoids were studied by Murota et al[21]. Quercetin, kaempferol and luteolin had strong free radical scavenging activity, whereas the isoflavone genistein and the flavone apigenin were poor radical scavengers. In another report, the antioxidant activities of a wide group of flavonoids (8 flavonols, 5 flavones, 2 flavonones, 3 isoflavones, 10 flavans and 1 flavanonol) were calculated from their oxidation potentials[22]. After comparing the antioxidant activities of different flavonoid species, the authors concluded that too much or too little lipophilicity in flavonoids hampers their antioxidant activity. Regarding the role of flavonoid structure in their antioxidant activity, it was emphasized that a crucial role is played by the 3-hydroxyl group. Also, other structures facilitating electron delocalization across the flavonoid molecule are important factors determining antioxidant activity. These structures include o-trihydroxyl groups, o-dihydroxyl groups, and the coexistence of 2,3-double bonds with 4-oxo and 3-hydroxyl groups.
The role of the number and position of hydroxyl groups with respect to the antioxidant activity of 5 flavonoids was investigated by Chen et al[23]. Apigenin did not protect canola oil against oxidation, presumably because of the presence of hydroxyl groups in positions 5', 7', and 4'. In contrast, the hydroxyl group at position 3', shared by kaempferol, morin, quercetin and myricetin, attenuated lipid oxidation. The greater antioxidant activity of quercetin relative to morin could be explained by the positions of their hydroxyl groups: adjacent hydroxyl groups in positions 3' and 4' were more effective than groups in the 2' and 4' positions. Similar experiments performed in erythrocyte membranes gave results similar to those recorded for canola oil.
It is necessary, however, to stress that in certain circumstances, flavonoids may also exert prooxidant effects. This flavonoid property is of some importance because it could contribute to tumor cell apoptosis and cancer chemoprevention. Autooxidation of flavonoids can be catalyzed by transition metals or by peroxidases[24]. Oxidation by flavonoids is thought to be involved in the inhibition of mitochondrial respiration or in flavonoid-induced hemoglobin oxidation and erythrocyte hemolysis.
The prooxidant activities of dietary flavonoids containing either phenol or catechol ring B were compared by Galati et al[24]. The catechol ring B containing the flavonoids luteolin, eriodicytol, and quercetin had lower redox potentials than their respective phenol ring B partners: apigenin, naringenin and kaempferol. The catechol ring B-containing flavonoids were more effective at oxidizing hepatocyte ascorbate than their phenol ring B equivalents. In general, the effectiveness of flavonoids at catalyzing the oxidation of ascorbate, β-nicotinamide adenine dinucleotide (NADH) and glutathione (GSH) had an inverse relationship with their redox potentials.
Influence of flavonoids on the structure and permeability of membranes
Oil-water or lipid-water partition coefficients are basic tools used to describe the ability of drugs to interact with biological membranes. Van Dijk et al determined the oil-water partition coefficients of several flavones, flavonols and flavanones[25]. As expected, the relative hydrophobicity of flavonoids depended on the number of hydroxyl groups. When flavones and flavanones possessing the same number of hydroxyl groups were compared it appeared that flavones were slightly more hydrophobic than flavanones. The affinity of tested flavonoids for liposomal membranes was determined by DPH fluorescence-quenching measurements. Despite the fact that flavanones are more hydrophobic than flavonols (at the same degree of hydroxylation), the membrane-affinity of flavanols was greater. This is presumably due to the planar structure of flavonols, which is preferred in the interactions of flavonoids with lipid bilayers. Surprisingly, glycosylation of naringenin and eriodicytol increased their affinity for liposomal membranes.
The Caco-2 cell line, derived from human colon adeno-carcinoma, is widely used as an in vitro model of absorption by epithelial cells because of its enterocyte-like characteri-stics. Using this cell line it is possible to follow the uptake and metabolism of different compounds. The importance of lipophilicity of isoflavone glucosides and aglycones for their uptake by Caco-2 cells and for interactions with phospholipid membranes was demonstrated by Murota et al[21]. It was shown that the isoflavone glucosides genistin and daidzin have lower affinity for PC vesicles than the aglycones genistein and daidzein. The flavonoids quercetin, kaem-pferol, luteolin and apigenin had strong affinities for the liposomal membranes. The membrane affinities of the flavo-noid aglycones were greater than those of the isoflavonoid aglycones. Murota et al have also shown that the membrane partitioning of the studied compounds correlated with their uptake and transport in Caco-2 cells.
The membrane affinity and permeability characteristics of flavonoids in Caco-2 and phospholipid vesicles were studied by Tammela et al[26]. The apical to basolateral flux of tested compounds was enhanced (with respect to the reference compounds ketoprofen and paracetamol) only by flavone-naringenin, which was transported in a similar way to reference compounds, whereas the other flavonoids studied ((+)-catechin, (-)-epicatechin, luteolin, morin, narigin and quercetin) were not transported in Caco-2 cells. The transport rates had an inverse relationship with the membrane affinity of the flavonoids [measured as the partition coefficient between 1-palmitoyl-2-oleoyl-sn-glycero-phospho-choline (POPC) vesicles and water], because strong membrane affinity was accompanied by poor transport in Caco-2 cells. A crucial role in the observed effects was played by the degree of hydroxylation of the flavonoid molecules.
The role of lipophilicity (related directly to the number of hydroxyl groups on ring B of the flavonoid moiety) in the toxicity of flavonols was studied by Kajiya et al[27]. Using 4 flavonols, galangin, kaempferol, quercetin and myricetin, they showed that the order of cytotoxicity against Chinese hamster lung fibroblast V79 cells matched the order of lipophilicity of these compounds. The exception was myricetin, which was least lipophilic but had the strongest cytotoxic effect. According to Kajiya et al, this exception was caused by the high rate of autooxidation of myricetin that occurred because of its unstable pyrogallol structure. Almost no hydrogen peroxide formation was observed for the other studied flavonols.
Black lipid bilayers (BLM) are a simple membrane model enabling the investigation of the electrical properties of bilayers. Using this model Movileanu et al studied the effects of insertion of quercetin into bilayers[28]. They showed that quercetin perturbs the structure of the lipid bilayer and increases its conductance and electrical capacitance. Penetration of the bilayer by quercetin molecules was pH-dependent (pH 5–8). The more acidic the medium, the more pronounced were the membrane capacitance changes that were observed. The authors of that paper suggested that hydrogen bonding between the 5-hydroxyl group and 4-oxygen forms the additional aromatic ring in the quercetin molecule.
Saija et al performed a calorimetric study on flavonoid-membrane interactions[29,30]. They demonstrated that the main phase transition temperature of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was shifted towards lower temperatures and that transition enthalpy remains practically unchanged upon the action of quercetin, hesperetin and naringenin. Rutin caused no detectable change in the character of the DPPC main phase transition. The temperature shifts were concentration-dependent. For the first 2 compounds, the effects were biphasic: for flavonoid:lipid mole fractions lower than 0.08 and 0.18 (for quercetin and hesperetin, respectively) the transition temperatures were lowered, but for higher mole fractions a subsequent increase in transition temperature was recorded. To explain these results it was proposed that the solubility of quercetin and hespertin in lipids may depend on the phase state of the bilayer. Also, a fluidizing effect of the flavonoids was assumed. Because this is the sole report claiming fluidization of the membrane structure by flavonoids, the authors’ arguments seem unconvincing.
Much more reliable results were obtained by Lehtonen et al[31] in their study on the binding of daidzein to liposomes. Using turbidity measurements they showed that daidzein induced liposome aggregation and that the extent of drug-induced turbidity depended on the type of lipid used to form liposomes. Charged lipid liposomes aggregated more strongly than those made of neutral phosphatidylcholine. The presence of cholesterol in liposomal membranes decreased the aggregation effects. According to the authors of this paper the aggregation is caused by a decrease in the hydration of the membrane surface induced by the presence of daidzein. Cholesterol increases the membrane hydration and therefore it prevents liposome aggregation.
Fluorescence polarization anisotropy measurements were used to study the localization and influence on membrane fluidity of a group of flavonoids and isoflavonoids[32]. A series of n-(9-anthroyloxy) fatty acids were used as fluorescent probes able to detect the position of the studied flavonoids in the lipid bilayer. All of the studied (iso)flavonoids (naringenin, rutin, genistein, genistin and biochanin A), as well as some flavonoid metabolites (equol, 4-hydroxy equol, dihydrodaidzein and dihydrogenistein) caused fluorescence polarization anisotropy increases, that is, a membrane fluidity decrease. The most pronounced fluidity changes induced by the presence of flavonoids in membranes were detected using the fatty acids with fluorescent moieties located close to the center of the bilayer, deep in the hydrophobic core. The effects exerted by flavonoids on membrane fluidity resemble those of cholesterol, and this membrane-stabilizing influence may contribute to the antioxidative properties of flavonoids.
Incorporation of flavonoids into the lipid bilayer is sometimes the first step in the sequence of events induced by polyphenolic compounds. Some isoflavonoids extracted from Sophora japonica caused the aggregation of liposomes composed either of neutral or of negatively charged lipids[33]. Strong liposome aggregation was induced by formononetin and irisolidone. The 6,8-diprenylgenistein caused weaker aggregation, whereas for licoisoflavone A, no aggregation effect was observed. Calorimetric measurements of the influence of these isoflavonoids on the thermotropic properties of DPPC revealed that presumably the compounds causing strong liposome aggregation were bound to the liposome surface, whereas 6,8-diprenylgenistein and licoisoflavone A penetrated the bilayer more deeply and were located close to the polar/apolar membrane interface. Because no significant differences were recorded when neutral or negatively charged liposome was used in aggregation experiments, the authors of this paper concluded that presumably the bilayer charge did not play important role in the interactions of the studied isoflavonoids and lipids. The mechanism of flavonoid-induced liposome aggregation proposed by Hendrich et al[33] is consistent with the model mechanism proposed for all polyphenols by Huh et al[34], who suggested the existence of polyphenolic bridges between adjacent surfaces. These bridges decrease hydration repulsion between bilayers and, because of hydrogen bonds, attract bilayer surfaces.
The incorporation of several isoflavones into liposomes, their partition between n-octanol/water phases, and cytotoxic activity against Chinese hamster lung fibroblast V79 cells were compared by Kato et al[35]. The main conclusion of this work was that the presence of the hydroxyl group at position 5 of ring A significantly enhances the lipophilicity of a flavonoid. This was illustrated by the example of 2 isoflavone pairs that differ exclusively by the presence (or absence) of this group, that is, genistein and daidzein, and biochanin A and formononetin. These findings seem to support the idea of Movileanu et al[28] that because of the hydrogen bonding between the 5-hydroxyl group and 4-oxygen, the additional aromatic ring is formed. The cytotoxic activities of the tested compounds followed their lipophilicity sequence.
The strength of the interactions of eight flavonoids with DPPC bilayers was found to be correlated with their ability to induce the leakage of calcein from small unilamellar egg yolk phosphatidylcholine (EYPC) liposomes by Olilla et al[36]. High performance liquid chromatography (HPLC) retention delay on a column coated with DPPC correlated with the number of hydroxyl groups in the tested flavonoids. Membrane calcein permeation induced by flavonoids depended on the lipophilicity of the studied compounds. The order of magnitude of calcein release induced by flavonoids was in the order acacetin>rhamnetin>apigenin>morin. Luteolin, quercetin and myricetin did not cause a significant calcein efflux from liposomes.
As in model liposomal membranes, the incorporation of flavonoids into cellular membranes decreases their fluidity, as shown in the case of apigenin and amentoflavone (api-genin homologous dimer)[37]. Amentoflavone causes much more pronounced fluidity changes than apigenin. Moreover, it has been proved that after interaction with the cellular membrane, amentoflavone is not internalized into the cell.
Because red blood cells are often used as a model in different types of experiments, the effects of flavonoids on the properties of erythrocyte membranes is an interesting research topic. Pawlikowska-Pawlega et al[38] studied the influence of quercetin on the properties of erythrocyte membrane by means of an electron spin resonance technique. Using spin labels located in different regions of the lipid bilayer, they showed that quercetin influences only the polar region of the bilayer, leaving the hydrophobic core of the membrane unchanged. This result confirms previous calorimetric data[39] obtained by the same research group. The characteristics of the DPPC main phase transition alteration induced by the presence of quercetin pointed to the localization of this flavone at the polar–apolar interphase of the lipid bilayer. Furthermore, it was shown that quercetin induced no hemolysis at concentrations of 1−25 µg/mL, but it caused the transformation of the erythrocyte shape[38]. At the highest concentration used (50 µg/mL), 47.7% of cells were transformed (into echinocyte-like shapes) and cell diameters were reduced. The authors concluded that quercetin localized close to the membrane surface protects it against peroxidation and, because of the induced changes in the structure of the bilayer, might cause alterations in its permeability.
The localization and distribution of 4 different flavonoid molecules in model POPC membranes was studied using nuclear magnetic resonance spectroscopy[40]. Using the chemical shift changes of the POPC signal induced by the presence of the flavonoid aromatic ring, the localization of flavone, chrysin, luteolin and myricetin molecules in lipid bilayers was calculated very precisely. According to these data, all lipid segments were influenced by the presence of flavonoids; however, the magnitude of the induced changes was greatest near the glycerol region. Depending on the flavonoid type, slight differences in their membrane positions were noticed. Flavone and chrysin were located closer to the bilayer center, whereas luteolin and myricetin distributions were biased towards the polar region of the bilayer. These data also suggest that distribution of flavonoids in the membrane is closely related to their polarity. The most apolar flavonoid was immersed deeper in the hydrophobic core of the bilayer and less polar flavonoids were located closer to the polar water phase. These results confirmed the conclusions of previous reports[31,33], except those of Arora et al[32], who postulated that flavonoid molecules were located deep inside the hydrophobic core of the bilayer. Analysis of the lateral diffusion coefficients of lipids and flavonoids showed that flavone, chrysin and luteolin were well incorporated into the bilayer and diffused laterally over the same time scale as lipids, whereas myricetin was more loosely bound with the bilayer and its diffusion resembled that of water molecules[40]. The authors concluded that flavonoids could reach all regions of the bilayer, and thus could prevent the whole bilayer from oxidation.
The influence of sophoraflavanone G (5,7,2',4'-tetra-hydroxy-8-lavandulylflavanone) on the fluidity of lipid bilayers was studied to elucidate the possible molecular mechanism of its intensive antibacterial activity[41]. Fluidity alterations were assessed using fluorescence polarization measurements. In both lipids used in the experiments, DPPC and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), a concentration-dependent increase of 1-anilinonaphthalene-8-sulfonic acid (ANS) and N-phenyl-1-naphthylamine (NPN) fluorescence polarization induced by the presence of sopho-raflavanone G was observed. The effects exerted on membrane fluidity were compared with those of naringenin, which appeared to have significantly weaker membrane rigidifying effects. Because fluorescence polarization changes were observed for both of the labels the used (ANS and NPN), the authors concluded that sophoraflavanone G and naringenin influenced hydrophilic as well as hydrophobic regions of the lipid bilayer. The fluidity changes observed in POPC systems are more likely to represent the situation in real bacterial membranes because of the high level of lipid unsaturation in those membranes. Antibacterial activity was also found for the isoflavones extracted from Lupinus argenteus[42].
The membrane rigidifying effects of a group of flavonoids were demonstrated by Tsuchiya et al[43,44]. By experimental methods similar to those of the work on sophoraflavanone G[41] it was shown that EGCg, genistein and apigenin decreased the fluidity of the lipid bilayers in a concentration-dependent manner[43]. Quercetin influenced the membrane fluidity in a biphasic fashion: at 5−10 µmol/L it decreased fluidity, whereas at lower concentrations (0.625−2.5 µmol/L) it increased fluidity. EGCg and apigenin affected both the hydrophobic and the hydrophilic regions of the bilayer, whereas genistein exclusively affected the hydrophobic part of the membrane. Daidzein failed to alter the fluidity of the studied lipid bilayers. The strongest effects in hydrophobic membrane regions were recorded for EGCg, whereas quercetin was the most effective at the membrane surface. The rigidifying effects were reduced when increasing amounts of cholesterol were added to the studied lipid systems. By increasing the ratio of unsaturated/saturated (18:1/16:0) phosphatidylcholine in bilayers, an increase in flavonoid rigidifying effects was observed. The authors concluded that an alteration of the membrane fluidity by flavonoids might generate multiple effects, leading to the final anticancer action of these compounds. Functions of membrane enzymes and receptors, as well as the reaction efficacy of membrane components could be modulated by fluidity changes.
Some compounds (for example ethanol) interact preferentially with the polar heads of lipids, and induce the appearance of a free space in the hydrocarbon chain region. This space is filled by the interpenetrating chains of lipids from the apposing lipid monolayer, and the so-called interdigitated structure is formed[45]. Some years ago flavonols were suggested as possible fluorescent membrane probes for the detection of interdigitation of lipid bilayers[46]. It was demonstrated that the fluorescence spectra of 3-hydroxy-4c-dimethylaminoflavone and 3-hydroxy-4'-(15-azacrown-5) flavone differ significantly when probes are located in interdigitated and noninterdigitated bilayers. 3-hydroxy-4-dimethylaminoflavone was additionally found to locate preferentially in the membrane domains rich in cholesterol.
Using a combination of centrifugation and HPLC, the affinity of several polyphenols for EYPC bilayers was compared[47]. Caffeic acid phenetyl ester, gallic acid lauryl ester, curcumin and quercetin had strong membrane affinities, whereas epicatechin, caffeic acid and gallic acid partitioned very poorly into the lipid bilayer. Like other reports concerning structure-activity relationships, this paper confirmed that compounds with strong membrane affinities were also biologically active at low concentrations.
Catechins are the flavonoids mostly found in green tea infusions. Tea flavonoids, and their chemical characterization and classification, have been thoroughly reviewed by Wang et al[48]. Like other flavonoids they are known to be antioxidative agents, and they also have anticarcinogenic and antibacterial activities. During research regarding the molecular mechanisms of their biological activity, the interactions between catechins and lipid bilayers were extensively studied. Using the methods previously applied in the study of the interactions of polyphenols with liposome membranes[47], Hashimoto et al studied the partition of several catechins into liposomes and the influence of these compounds on the permeability of liposomal membranes for calcein[49]. It was found that the affinities of catechin gallic esters (ECg and EGCg) for the lipid bilayers were much greater than the affinities of the corresponding catechins [epicatechin (EC) and epigallocatechin (EGC), respectively]. A similar difference between catechins and catechin gallic esters was found when the influence of these compounds on calcein leakage from liposomes was studied. Catechins had apparently no effect on the efflux of calcein trapped inside EYPC liposomes, whereas their gallic esters inhibited the efflux in a concentration-dependent manner. The difference between the effects exerted on membranes by gallate esters and nonesters of catechins was confirmed also in a study on the influence of such compounds on membrane fluidity[50] (measured by ANS and NPN fluorescence polarization anisotropy). The gallate esters EGCg, GCg, ECg and Cg decreased membrane fluidity (measured as an increase in the fluorescence polarization of a fluorescent label incorporated into the membrane) more significantly than the corresponding nonesters. Such a reduction in membrane fluidity may be associated with the hepatoprotective effects of catechins.
Contrary to the conclusions presented above[49,50], no influence of catechin and epicatechin on membrane fluidity was found by Verstraeten et al[51]. Both catechins used in that work protected liposomes against detergent-induced disruption and inhibited lipid oxidation. The membrane protective properties of catechins were attributed to their interactions with lipid polar heads, particularly with those containing hydroxyl groups.
Using fluorescence spectroscopy Hashimoto et al also showed that after incorporation catechins are located on the surface of liposome membranes[49]. Comparison of these data with the biological activity of the studied compounds (in vitro lung cancer growth inhibition, reduction of LDL oxidation) revealed that the ability of catechins to interact with lipids plays presumably an essential role in the mechanisms of the observed biological effects.
Further studies have shown also that steric effects are of some importance when the interactions of catechins with lipid membranes is considered[52]. Depending on the configuration of the 2 hydrogens at positions 2 and 3 of the ring C (Figure 1) cis- and trans- types of catechins are distingui-shed. Membrane-affinity studies have shown that cis-type catechins incorporate into lipid bilayers in greater amounts than trans-type catechins. Partition coefficients between n-octanol and buffer of cis-type catechins were greater than for the corresponding trans-type catechins. All of the catechins studied in this work also affected calcein leakage from liposomes. In lower concentrations (up to 1 nmol/L) a decrease in efflux was observed, whereas for higher concentrations a gradual increase in calcein leakage from liposomes was recorded. Despite of the similarity of the observed effects, the magnitude of the effect was smaller for trans-type catechins than for the cis-type ones.
Stereospecifity in the interactions of cis- and trans- types of catechins with DPPC, DOPC, and POPC bilayers was studied by Tsuchiya[53]. Using fluorescence polarization spectroscopy Tsuchiya compared the effects of catechin stereoisomers on the fluidity of lipid bilayers. In most cases the cis-catechins (+)-EC and (-)-EC decreased membrane fluidity to a greater extent than did their trans-stereoisomers ((+)-C and (-)-C, respectively). The effects of catechin stereoisomers depended on the type of lipid used in the experiment. For DOPC and POPC liposomes the catechin-induced fluidity decrease was approximately 2 times smaller than for DPPC liposomes. The addition of 5% of cholesterol increased the differences between the effects evoked by cis- and trans- catechin stereoisomers. The application of different types of fluorescent labels enabled Tsuchiya to localize the acting sites of catechins in membranes[53]. Tsuchiya concluded that (-)-EC presumably acted closer to the membrane core whereas (+)-C acted near the bilayer surface.
A detailed study of catechin-lipid interactions was presented by Caturla et al[54]. Using differential scanning calorimetry, infrared spectroscopy and fluorescence polarization anisotropy measurements, they investigated the interaction of C, EC, ECg and EGCg with membranes composed of phosphatidylcholine or phosphatidylethanolamine. The catechins with galloyl moieties partition into the lipid bilayers and perturb their structure much easier than their nongalloylated partners. The lipid-water partition coefficients of galloyl catechin derivatives were also much greater than those of nongalloylated catechins (2.92×104 and 1.92×104 for ECg and EGCg, respectively vs 0.22×104 and 0.2×104 for EC and C, respectively). Measurements of catechin intrinsic fluorescence quenching by 5-NS and 16-NS spin probes revealed that ECg is located deeper in the hydrocarbon chain region of the bilayer than epigallocatechin gallate (EGCG). As was already found by other research groups Caturla et al also recorded a decrease in membrane fluidity induced by ECg and EGCg. Formation of compact and rigid structures was followed by the appearance of local defects that increased the permeability of the liposome membrane to calcein. Membrane permeation and damage induced by catechins were previously proposed as putative mechanisms of the antibacterial action of green tea flavonoids, particularly EGCg[55].
The incorporation of catechins into lipid bilayers can be influenced by such factors as salt concentration in the medium or the electric charge of the membrane[56]. An increase in the amount of catechins (EC, EGCg, ECg) incorporated into the liposome membranes was observed when the salt concentration in the phosphate-buffered saline (PBS) buffer was increased. Thus the salting-out effect of the aqueous medium can be important for the in vitro activity of catechins. The negative electric charge of the membrane (achieved by the addition of 10% egg phosphatidylserine) decreased significantly the number of catechins (EC, EGCg, ECg) incorporated into the liposome membranes. This effect could be responsible for the resistance of Gram-negative bacteria to catechins, because these bacteria have negatively charged lipopolysaccharides on the external side of the membrane. The presence of a positive charge in the membrane (the addition of 10% stearylamine) did not change the incorporation of catechins into liposome membranes.
Using fluorescence spectroscopy Kitano et al[57] showed that EGCg incorporates into POPC, DOPC, and DMPC bilayers. The results of this work confirmed the findings of Hashimoto et al[49] that in phosphatidylcholine bilayers EGCg is located at the membrane surface. By measuring the partitioning of EGCg into 3 different phospholipid bilayers these authors found that the affinity of (-)-epigallocatechin gallate for the membranes increases in the order DMPC<POPC&DOPC corresponding thus to the loosening of the lipid molecules packing in bilayer. It was also shown in this work that EGCg inhibits PKC activation in the presence of DMPC liposomes. The authors suggested that this inhibition is caused by the perturbation of the membrane structure induced by EGCg.
Flavonoids as anticancer agents and multidrug resistance modifiers
The term “multidrug resistance” (MDR) is used to describe the ability of cells exposed to a single drug to develop resistance to a broad range of structurally and functionally unrelated drugs[58]. Such resistance can be achieved by cancer cells as well as by bacteria and yeast. In all cases MDR constitutes a major obstacle in the (chemo)therapy of a disease. Several mechanisms are involved in the MDR phenomenon: interference with apoptosis, overexpression of transport proteins that extrude drugs from the cell or alteration of enzyme activity (ie glutathione transferase or topoisomerase); for review see Krishna and Mayer[59]. Without doubt, the active outward transport of drugs by ATP-driven membrane proteins such as P-gp (ABCB1), MRP1 (ABCC1) or BCRP (ABCG2) is the most important MDR mechanism[60–62]. ABCB1, ABCC1 and ABCG2 are members of the superfamily of ATP binding cassette protein (ABC) proteins. Considering drug accumulation within the cell, the role of passive inward drug flow must also be taken into account. The balance between passive inward and active outward drug fluxes determines if the drug is accumulated inside the cell or not[63,64]. It is important to emphasize that both these fluxes can be regulated by the alteration of biophysical properties of the lipid bilayer, mainly by its fluidity. Because passive influx of the drug is just simple diffusion[65], it is obvious that it depends on membrane properties. On the other hand, it is also known that the activity of membrane transport proteins like P-gp can be modulated by the physical state of the surrounding lipids[66,67], as well as by the lipid bilayer composition[68]. The role of lipid bilayer in MDR and its modulation has been recently reviewed by Hendrich and Michalak[69].
MDR can be reversed or circumvented when, in parallel to chemotherapy, so-called chemosensitizers or modulators are co-applied[58,70]. The first known MDR modulator was verapamil[71] but since its discovery the list of chemosensitizers has become very long, and now contains substances such as phenothiazines, calcium antagonists, indole alkaloids, cyclosporins, flavonoids and many others[59].
P-gp and MRP1 are not present exclusively in the membranes of cancer cells, they also occur in normal human tissues including brush border, adrenal cortex, kidney proximal convoluted tubules, many types of epithelial cells, cells at the blood-brain barrier, and others. The physiological function of these ABC transporters is the clearance of natural cytotoxic compounds from the cells. Therefore, P-gp and MRP1 present in normal tissues can contribute to defense against naturally occurring carcinogens. Flavonoids have been found to exert a differentiated influence on the transport activity of ABCB1 protein[72]. Adriamycin accumulation in HCT-15 colon cells was inhibited most effectively by the flavonols galangin, kaempferol and quercetin. Flavonoids belonging to other tested groups, the flavones, flavanones, chalcones, isoflavones and flavanols, were less efficient adriamycin accumulation inhibitors, or had no inhibitive activity at all. The same flavonoids that attenuated drug accumulation were found to significantly accelerate adriamycin efflux from cancer cells. Because the flavonoid-induced drug efflux could be stopped by verapamil, there were no doubts that P-gp was involved in adriamycin efflux.
The effects of 4 flavonoids, biochanin A, morin, phloretin and silymarin, on P-gp (ABCB1)-mediated transport in drug-resistant and drug-sensitive breast cancer cells were studied by Zhang and Morris[73]. It was found that all flavonoids increased the accumulation of daunomycin in cancer cells overexpressing P-gp. Not all flavonoids, however, increased the cytotoxicity of doxorubicin in the studied cells. Biochanin A and silymarin were able to increase doxorubicin toxicity, whereas morin and phloretin showed no effect. To elucidate the mechanism of the increase in drug accumulation, 3 possibilities were investigated. The first one was an assumption that flavonoids alter the passive permeability of cancer cells to drugs. It was shown, however, that none of the tested flavonoids increased the uptake of daunomycin in drug-sensitive cells (not overexpressing P-gp). The second possibility was that the ATPase activity of P-gp is reduced by those flavonoids that induce drug accumulation. This mechanism was definitely excluded because depending on the type of flavonoid, ATPase activity was either increased (by biochanin A and phloretin) or decreased (by silymarin). Because biochanin A and silymarin both increase doxorubicin toxicity, the opposite effects they exert on ATPase activity could not explain the observed accumulation effects. The third studied mechanism was competitive flavonoid binding to the drug binding site on the P-gp molecule. Also in this case no clear picture was obtained: biochanin A and phloretin bound only weakly to the P-gp substrate binding site, whereas silymarin and morin were bound to a much greater extent.
Shapiro and Ling[74] showed that inhibition of the P-glycoprotein-mediated transport of Hoechst 33342 by quercetin is at least partially caused by the inhibition of the ATPase activity of this protein. According to these authors, the previously reported stimulatory effect of quercetin on drug efflux from multidrug resistant cells was due to the indirect effect exerted on P-glycoprotein.
A comprehensive review devoted to MDR modulation by flavonoids has been written by Di Pietro et al[75]. In this paper the interactions of flavonoids with P-gp as well as with other MDR-related transporters (Leishmania Ltrmdr1, yeast Pdr5p) are described. Among other topics, the role of prenylation of flavonoid molecules was thoroughly discussed and a model of prenylated- and non-prenylated flavonoid molecules binding to P-glycoprotein was presented.
The affinity of a huge number of flavonoids for the nucleotide binding domain (NBD) of P-glycoprotein was reviewed by Boumendjel et al[76]. This review covers several flavonoid groups: the chalcones, flavonols, C-alkylated flavones, flavanones and prenylated xanthones. Some conclusions concerning the structure-activity relationships were also included in the last paragraph of this review. As found in antioxidation studies, the strongest NBD-binding properties are possessed by flavonoids hydroxylated at the 3 and 5 positions, possessing the 4-carbonyl group or a 2–3 double bond.
MRP1 (ABCC1) is, like P-gp, a multidrug transporter protein, contributing to the reduction of the intracellular accumulation of anticancer drugs in cancer cells. Substrates for MRP1 are organic anions that for transport purposes are conjugated with glutathione, glucuronide or sulfate[77,78]. MRP1 is expressed in the majority of tissues in the human body, and it occurs also in several types of tumor cells. There are several potential mechanisms of MDR reversal, some of which may involve direct or indirect (via the lipid phase of the membrane) interactions with chemosensitizing agents.
GSH-conjugated leukotriene C4 is a well known MRP1 substrate, and transport of this conjugate is used to characterize the transport activity of ABCC1. The influence of some flavonoids on the MRP1-mediated transport of leukotriene C4 was studied by Leslie et al[79]. They found that addition of GSH enhanced the inhibition of lekotriene C4 transport by genistein, apigenin and naringenin, whereas other flavonoids studied, myricetin, quercetin and kaempferol, were potent transport inhibitors even without GSH. The inhibition enhancement induced by GSH seems to result from the increased affinity of transport inhibitors for the protein in the presence of glutathione. The inhibitory potency of flavonoids is presumably not directly related to their hydrophobicity, because the MRP1 inhibition order does not match the hydrophobicity order (as determined by reverse phase column HPLC). The inhibition of leukotriene C4 transport by flavonoids seems to be competitive: flavonoids bind to the same binding sites on MRP1 as transport substrates. However, the flavonoids bind to more than one binding site on MRP1 because at least in the case of quercetin binding to NBD, the domain was also proved. Unexpectedly, the most potent leukotriene C4 transport inhibitors (apigenin and naringenin) were also the most potent stimulators of GSH transport. The authors of this report had not yet found any proof that flavonoids were co-transported with GSH by MRP1. Further studies performed by this research group revealed that apigenin, naringenin, genistein and quercetin-stimulated GSH transport may lower the GSH content of the MRP1-overexpressing cells, but that this effect can be at least partially balanced by increased glutathione biosynthesis[80].
Because flavonoids are known as anticancer agents, the ability to inhibit transport by MRP1 of a set of 22 dietary flavonoids was studied[81]. In order to determine the ability of flavonoids to alter MRP1 activity, their influence on daunomycin and vinblastine accumulation in a human pancreatic adenocarcinoma cell line was measured. Accumulation of daunomycin was increased when cells were treated with 100 μmol/L of morin, biochanin A, chalcone, sylimarin, phloretin, genistein, quercetin, chrysin and kaempferol, whereas drug accumulation was decreased by apigenin, fisetin, galangin, luteolin, myricetin and rhoifolin. Vinblastine accumulation in cancer cells was generally greater than that observed with daunomycin. It was increased by morin, biochanin A, chalcone, sylimarin, phloretin, genistein, quercetin, apigenin, epigallocatechin and kaempferol. Fisetin and rutin decreased the vinblastine accumulation in cancer cells. A significant correlation between the inhibition of daunomycin and vinblastine transport induced by different flavonoids was observed. In an experiment on the influence of flavonoids on MRP1 expression in cancer cells, no significant effects of biochanin A, chalcone, phloretin, quercetin, morin, genistein, rhoifolin or sylimarin were recorded. All of the flavonoids that increased drug accumulation in cancer cells (except morin) also produced a decrease in cellular GSH concentra-tion.
The influence of the series of 22 flavonoids on the MRP1-mediated transport of daunomycin and vinblastine was studied using Panc-1 cells as a model expressing the transport protein[81]. It was found that biochanin-A, genistein, quercetin, chalcone, silymarin, phloretin, morin and kaempferol significantly increased daunomycin and vinblastine accumulation in Panc-1 cells, when they were used at a concentration of 100 µmol/L. Simultaneously, these flavonoids had no effect on MRP1 membrane expression or S-glutathione transferase activity.
Inhibition of MDR proteins MRP1 and MRP2 by a series of 29 flavonoids was studied recently by van Zanden et al[82]. It was shown that MRP1 was more susceptible to inhibition by flavonoids than MRP2. The results of inhibition experiments together with some molecular structure descriptors were used to define the structural requirements of flavonoids necessary for effective inhibition of MRP1- and MRP2-mediated transport. It appeared that the following features were optimal for MRP1 inhibition: the presence of methoxyl and hydroxyl groups in flavonoid molecules, as well as the dihedral angle between B- and C- flavonoid rings. Regarding MRP2 inhibition, only the presence of a B-ring pyrogallol group seemed to be an important structural factor.
MRP1 is expressed also in red blood cell membranes and therefore erythrocytes can be used as a simple model in MRP1-mediated transport studies[83,84]. Using this model, Bobrowska-Haegerstrand et al studied the influence of several membrane-perturbing agents (including the isoflavones genistein, daidzein, licoisoflavone A and sophoraisoflavone A) on the transport activity of MRP1[85,86]. Except for daidzein, all isoflavones tested decreased the fluorescent dye efflux from erythrocytes and thus inhibited MRP1 transport activity. At the concentrations used in the experiments no red blood cell shape alteration was recorded and no hemolysis was observed.
ABC proteins transport their substrates using the energy obtained from ATP hydrolysis. Therefore, the factors interfering with the ATPase activity of these proteins may play an important role in MDR modulation. Because some studies have found that flavonoids bind to NBD sites[87], it seemed reasonable to investigate the influence of those compounds on the ATPase activity of transport proteins (ABCB1 and ABCC1). Hooijberg et al studied the influence of 4 flavonoids on the ATPase activity of the MRP1 protein[88]. Three of the tested (iso)flavonoids (genistein, kaempferol and flavopiridol) stimulated the ATPase activity because of the direct interaction with MRP1. Genistin (a glucosylated derivative of genistein) did not affect the ATPase activity of MRP1, and simultaneously has not inhibited the dauno-rubicin transport mediated by MRP1. These facts led to the conclusion that certain flavonoids interact directly with the substrate-binding site of MRP1.
High-affinity binding of several classes of flavonoids with NBD2 from mouse P-glycoprotein was shown by Conseil et al[89]. Using the intrinsic fluorescence of single tryptophan residues they showed that flavones (quercetin, apigenin) bind to NBD2 more strongly than flavanones (naringenin) and isoflavones (ganistein). All flavonoids studied overlap with respect to the ATP binding site and also the vicinal hydrophobic protein region interacting with steroids. This finding at least partially explains the mechanism of P-gp modulation by flavonoids.
Also, catechins were found to interact with P-gp and to affect the transport activity of this protein[90]. Some of the catechins inhibited the transport of fluorescent markers by ABCB1 and others significantly elevated transport by P-gp. Inhibition or acceleration effects depended not only on the type of catechin used but also on the kind of fluorescent marker. It was concluded that heterotropic allosteric enhancement of P-gp can explain the anticarcinogenic properties of green tea catechins.
The existence of multiple flavonoid binding sites within MRP1 was demonstrated by Trompier et al[87]. Using the recombinant nucleotide-binding domains from human MRP1 overexpressed in bacteria, they showed that flavonoids exhibit high affinity for NBD1 and NBD2 and compete with ATP for binding. The NBD binding affinity order was as follows: flavonols (quercetin, galangin)>flavones (apigenin), flavonones (naringenin)>isoflavones (genistein). Additionally, it was found that isoprenylation of flavonoid molecules leads to the preferential binding of such molecules with NBD1 (in comparison with NBD2).
Influence of flavonoids on ionic channels and membrane conductivity
As was reviewed in previous sections, flavonoids interact with lipid bilayers as well as with membrane proteins. It is thus obvious that such interactions must influence also the electric properties of membranes. In this section only a few examples of flavonoid interactions with ionic channels will be given.
In the mid-1970s it was found that phloretin dramatically alters anion and cation conductance in membranes containing ion carriers[91]. In phosphatidylethanolamine membranes containing nonactin, K+ conductance was increased 105-fold, whereas in the same membranes containing carbonyl-cyanide-m-chlorophenylhydrazone as anion carriers, the conductance for tetraphenylborate was decreased 103-fold by 100 µmol/L of phloretin. These effects were explained by the alteration of membrane dipolar potential induced by the absorption of phloretin molecules (possessing large dipole moment) on the membrane surface. The newly established electric field prevents the passage of anions and accelerates the passage of cations through the membrane.
As was demonstrated by Okamoto et al, potassium currents carried by inward rectifier K+ channels are inhibited by genistein in osteoclasts[92]. Using the whole-cell patch-clamp technique these researchers showed that physiologically attainable concentrations of genistein applied extracellularly inhibit potassium currents in a concentration-dependent manner. Also, daidzein had weak inhibitory properties. The osteoclast membrane depolarization caused by genistein can inhibit the bone resoprtion process and in this way the flavonoid may exert its bone-protective effects. Inhibition of inward rectifying potassium current in guinea pig ventricular myocytes by genistein and daidzein was recorded by Chiang et al using the patch-clamp and voltage clamp techniques[93]. This inhibition may be responsible for the clinically observed pro-arrhythmic effects of genistein.
In contrast to the isoflavone genistein, the flavonol quercetin induced activation of the basolateral K+ channels in rat distal colon epithelium membranes[94]. This K+ channel activation seemed to be responsible for the previously detected quercetin-induced chloride secretion in rat colon[95].
Quercetin activates the skeletal muscle Ca2+ release channels (ryanodine receptors) as was shown by Lee et al[96]. Statistical analysis of single channel currents, recorded using rabbit skeletal ryanodine receptors reconstituted into planar lipid bilayers, revealed that in the presence of 10 mmol/L Ca2+, quercetin increased the probability of channels opening in a concentration-dependent manner. A synergistic effect of quercetin and caffeine on single ryanodine receptors was also found.
Genistein was found to interact with the cystic fibrosis transmembrane conductance regulator (CFTR) channels and activate them at low concentrations[97,98] or inhibit the activity of this protein in high concentrations (above 50 µmol/L)[99]. Initially, it was proposed that genistein indirectly stimulates channel activity by preventing CFTR inhibition by protein tyrosine kinase[98]. More recent reports, however, point to the direct interaction of genistein with the channel protein[99]. As in the case of other ABC transporter proteins[87,89] flavonoid molecules bind to the NBD2 domain of phosphorylated channel protein and inhibit the ATPase activity of the nucleotide binding site[100].
Conclusion
Flavonoids are consumed with food as well as in the form of flavonoid-containing dietary supplements or herbal products. Increasing knowledge about their beneficial health effects is causing increased flavonoid consumption. However, for many years it has been known that the spectrum of effects exerted by flavonoids is very broad. Therefore, careful evaluation of the biological activity of flavonoids seems to be important for the proper determination of their safety.
As was shown in the present review, flavonoids can interact with both lipid and protein components of biological membranes and alter their properties. Interactions with lipids are, in most cases, limited to the polar region of the lipid bilayer; however, the depth of membrane penetration by flavonoids depends on their structure. Most flavonoids studied decrease membrane fluidity. The main factor governing the strength of flavonoid-lipid interactions seems to be the lipophilicity of the flavonoid molecules. It seems also that the presence of the 3-hydroxyl group and the 2,3 double bond are of utmost importance for the antioxidative activity of flavonoids.
Flavonoids interact also with membrane transporter proteins and thus inhibit the drug transport responsible for MDR. The structural features of flavonoids that are propitious for their inhibitory properties are almost the same as those that bring about their antioxidative activity. The presence of 3- or 5-hydroxyl groups and the 2,3 double bond are also important. In some cases prenylation of the flavonoid molecule increases its inhibitory potency.
Knowledge about flavonoid interactions with membrane components seems to be sufficient to predict the structure of potential flavonoid-based drugs and to harness the desired biological effects. The same knowledge should allow the possible side-effects associated with flavonoid usage to be minimized.
References
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry 2000;55:481-504.
- Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther 2002;96:67-202.
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 2002;13:572-84.
- Barron D, Ibrahim RK. Isoprenylated flavonoids: a survey. Phytochemistry 1996;43:921-82.
- Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Invest 2002;22:51-65.
- Brandi ML. Natural and synthetic isoflavones in the prevention and treatment of chronic diseases. Calcif Tissue Int 1997;61:S5-S8.
- Le Marchand L. Cancer preventive effects of flavonoids: a review. Biomed Pharmacother 2002;56:296-301.
- Mizunuma H, Kanazawa K, Ogura S, Otsuka S, Nagai H. Anticarcinogenic effects of isoflavones may be mediated by genistein in mouse mammary tumor virus-induced breast cancer. Oncology 2002;62:78-84.
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrynology 1998;139:4252-63.
- Sadzuka Y, Sugiyama T, Shimoi K, Kinae N, Hirota S. Protective effect of flavonoids on doxorubicin-induced cardiotoxicity. Toxicol Lett 1997;92:1-7.
- Yamaguchi M, Gao YH. Inhibitory effect of genistein on bone resorption in tissue culture. Biochem Pharmacol 1998;55:71-6.
- Dragsted LO. Antioxidant actions of polyphenols in humans. Int J Vitam Nutr Res 2003;73:11-9.
- Muscatello U, Alessandrini A, Valdre G, Vannini V, Valdre U. Lipid oxidation deletes the nanodomain organization of artificial membranes. Biochem Biophys Res Commun 2000;270:448-52.
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 2000;275:17221-4.
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct 2003;32:257-83.
- Gordon MH., Roedig-Penman A. Antioxidant activity of quercetin and myricetin in liposomes. Chem Phys Lipids 1998;97:79-85.
- Arora A, Nair MG, Straasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys 1998;356:133-41.
- van Acker SA, van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ, et al. Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med 1996;20:331-42.
- Liao K, Yin M. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: importance of the partition coefficient. J Agric Food Chem 2000;48:2266-70.
- Madsen HL, Andersen CM, Jorgensen LV, Skibsted LH. Radical scavenging by dietary flavonoids. A kinetic study of antioxidant efficiencies. Eur Food Res Technol 2000;211:240-6.
- Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, et al. Unique uptake and transport of isoflavone aglycones by human intestinal caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr 2002;132:1956-61.
- Yang B, Kotani A, Arai K, Kusu F. Estimation of the antioxidant activities of flavonoids from their oxidation potentials. Anal Sci 2001;17:599-604.
- Chen ZY, Chan PT, Hoa KY, Funga KP, Wang J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem Phys Lipids 1996;79:157-63.
- Galati G, Sabzevari O, Wilson JX, O’Brien PJ. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002;177:91-104.
- van Dijk C, Driessen AJ, Recourt K. The uncoupling efficiency and affinity of flavonoids for vesicles. Biochem Pharmacol 2000;60:1593-600.
- Tammela P, Laitinen L, Galkin A, Wennberg T, Heczko R, Vuorela H, et al. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch Biochem Biophys 2004;425:193-9.
- Kajiya K, Ichiba M, Kuwabara M, Kumazawa S, Nakayama T. Role of lipophilicity and hydrogen peroxide formation in the cytotoxicity of flavonols. Biosci Biotechnol Biochem 2001;65:1227-9.
- Movileanu L, Neagoe I, Flonta ML. Interaction of the antioxidant flavonoid quercetin with planar lipid bilayers. Int J Pharm 2000;205:135-46.
- Saija A, Bonina F, Trombetta D, et al. Flavonoid-biomembrane interactions: a calorimetric study on dipalmitoylphospha-tidylcholine vesicles. Int J Pharm 1995;124:1-8.
- Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavanoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med 1995;19:481-6.
- Lehtonen JY, Adlercreutz H, Kinnunen PK. Binding of daidzein to liposomes. Biochim Biophys Acta 1996;1285:91-100.
- Arora A, Byrem TM, Nair MG., Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys 2000;373:102-9.
- Hendrich AB, Malon R, Pola A, Shirataki Y, Motohashi N, Michalak K. Differential interaction of Sophora isoflavonoids with lipid bilayers. Eur J Pharm Sci 2002;16:201-8.
- Huh NW, Porter NA, McIntosh TJ, Simon SA. The interaction of polyphenols with bilayers: conditions for increasing bilayer adhesion. Biophys J 1996;71:3261-77.
- Kato R, Kajiya K, Tokumoto H, Kumazawa S, Nakayama T. Affinity of isoflavonoids for lipid bilayers evaluated with liposomal systems. Biofactors 2003;19:179-87.
- Ollila F, Halling K, Vuorela P, Vuorela H, Slotte JP. Characterization of flavonoid–biomembrane interactions. Arch Biochem Biophys 2002;399:103-8.
- Lenne-Gouverneur AF, Lobstein A, Haan-Archipoff G, Duportail G, Anton R, Kuhry JG. Interactions of the monomeric and dimeric flavones apigenin and amentoflavone with the plasma membrane of L929 cells; a fluorescence study. Mol Membr Biol 1999;16:157-65.
- Pawlikowska-Pawlêga B, Gruszecki WI, Misiak LE, Gawron A. The study of the quercetin action on human erythrocyte membranes. Biochem Pharmacol 2003;66:605-12.
- Wójtowicz K, Pawlikowska-Pawlêga B, Gawron A, Misiak LE, Gruszecki WI. Modifying effect of quercetin on the lipid membrane. Folia Histochem Cytobiol 1996;34:49-50.
- Scheidt HA, Pampel A, Nissler L, Gebhardt R, Huster D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. Biochim Biophys Acta 2004; 1663: 97–107.
- Tsuchiya H, Iinuma M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 2000;7:161-5.
- Morel C, Stermitz FR, Tegos G, Lewis K. Isoflavones as potentiators of antibacterial activity. J Agric Food Chem 2003;10:5677-9.
- Tsuchiya H, Nagayama M, Tanaka T., Furusawa M, Kashimata M, Takeuchi H. Membrane-rigidifying effects of anti-cancer dietary factors. Biofactors 2003;16:45-56.
- Furusawa M, Tsuchiya H, Nagayama M, Tanaka T, Nakaya K, Iinuma M. Anti-platelet and membrane-rigidifying flavonoids in brownish scale of onion. J Health Sci 2003;49:475-80.
- Simon SA, McIntosh TJ. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspension. Biochim Biophys Acta 1984;773:169-72.
- Bondar OP, Pivovarenko VG, Rowe ES. Flavonols: new fluorescent membrane probes for studying the interdigitation of lipid bilayers. Biochim Biophys Acta 1998;1369:119-30.
- Nakayama T, Ono K, Hashimoto K. Affinity of antioxidative polyphenols for lipid bilayers evaluated with a liposome system. Biosci Biotech Biochem 1998;62:1005-7.
- Wang H, Provan GJ, Helliwell K. Tea favonoids: their functions, utilisation and analysis. Trends Food Sci Technol 2000;11:152-60.
- Hashimoto T, Kumazawa S, Nanjo F, Hara Y, Nakayama T. Interaction of tea catechins with lipid bilayers investigated with liposome systems. Biosci Biotechnol Biochem 1999;63:2252-5.
- Tsuchiya H. Effects of green tea catechins on membrane fluidity. Pharmacology 1999;59:34-44.
- Verstraeten SV, Keen CL, Schmitz HH, Fraga CG, Oteiza PI. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic Biol Med 2003;34:84-92.
- Kajiya K, Kumazawa S, Nakayama T. Steric effects on interaction of tea catechins with lipid bilayers. Biosci Biotechnol Biochem 2001;65:2638-43.
- Tsuchiya H. Stereospecificity in membrane effects of catechins. Chem Biol Interact 2001;134:41-54.
- Caturla N, Vera-Samper E, Villalain J, Mateo CR, Micol V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic Biol Med 2003;34:648-62.
- Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta 1993;1147:132-6.
- Kajiya K, Kumazawa S, Nakayama T. Effects of external factors on the interaction of tea catechins with lipid bilayers. Biosci Biotechnol Biochem 2002;66:2330-5.
- Kitano K, Nam KY, Kimura S, Fujiki H, Imanishi Y. Sealing effects of (-) - epigallocatechin gallate on protein kinase C and protein phosphatase 2A. Biophys Chem 1997;65:157-64.
- Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev 1990;42:155-99.
- Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 2000;11:265-83.
- Gottesman MM, Pastant I, Ambudkar SV. P-glycoprotein and multidrug resistance. Curr Opinion Genet Devel 1996;6:610-7.
- Blackmore CG, McNaughton PA, Van Veen HW. Multidrug transporters in prokaryotic and eukaryotic cells: physiological functions and transport mechanisms. Mol Membr Biol 2001;18:97-103.
- Sugimoto Y, Tsukahara S, Ishikawa E, Mitsuhashi J. Breast cancer resistance protein: Molecular traget for anticancer drug resistance and pharmacokinetics/pharmacodynamics. Cancer Sci 2005;96:457-65.
- Eytan GD, Regev R, Oren G, Assaraf YG. The role of passive transbilayer drug movement in multidrug resistance and its modulation. J Biol Chem 1996;271:12897-902.
- Eytan GD, Kuchel PW. Mechanism of action of P-glycoprotein in relation to passive membrane permeation. Int Rev Cytol 1999;190:175-250.
- Frezard F, Garnier-Suillerot A. Comparison of the membrane transport of anthracycline derivatives in drug-resistant and drug-sensitive K562 cells. Eur J Biochem 1991;196:483-91.
- Sinicrope FA, Dudeja PK, Bissonnette BM, Safa AR, Brasitus TA. Modulation of P-glycoprotein-mediated drug transport by alterations in lipid fluidity of rat liver canalicular membrane vesicles. J Biol Chem 1992;267:24995-5002.
- Dudeja PK, Anderson KM, Harris SH, Buckingham L, Coon JS. Reversal of multidrug resistance phenotype by surfactants: relationship to membrane lipid fluidity. Arch Biochem Biophys 1995;319:309-15.
- Romsicki Y, Sharom FJ. The membrane lipid environment modulates drug interactions with the P-glycoprotein multidrug transporter. Biochemistry 1999;38:6887-96.
- Hendrich AB, Michalak K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr Drug Targ 2003;4:23-30.
- Ford JM. Experimental reversal of P-glycoprotein-mediated multidrug resistance by pharmacological chemosensitisers. Eur J Cancer 1996;32A:991-1001.
- Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res 1981;41:1967-72.
- Critchfield JW, Welsh CL, Phang JM, Yeh GC. Modulation of adriamycin accumulation and efflux by flavonoids in CHT-15 colon cells. Biochem Pharmacol 1994;48:1437-45.
- Zhang S, Morris ME. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther 2003;304:1258-67.
- Shapiro AB, Ling V. Effect of quercetin on Hoechst 33342 transport by purified and reconstituted P-glycoprotein. Biochem Pharmacol 1997;53:587-96.
- Di Pietro A, Conseil G, Perez-Victoria JM, Dayan G, Baubichon-Cortay H, Trompier D, et al. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell Mol Life Sci 2002;59:307-22.
- Boumendjel A, Di Pietro A, Dumontet C, Barron D. Recent advances in the discovery of flavonoids and analogs with high-affnity binding to P-glycoprotein responsible for cancer cell multidrug resistance. Med Res Rev 2002;22:512-29.
- Hipfner DR, Deeley RG, Cole SP. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta 1999;1461:359-76.
- Loe DW, Almquist KC, Deeley RG, Cole SP. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem 1996;271:9675-82.
- Leslie EM, Mao Q, Oleschuk CJ, Deeley RG, Cole SP. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and ATPase activities by interaction with dietary flavonoids. Mol Pharmacol 2001;59:1171-80.
- Leslie EM, Deeley RG, Cole SP. Bioflavonoid stimulation of glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1). Drug Metab Dispos 2003;31:11-5.
- Nguyen H, Zhang S, Morris ME. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J Pharm Sci 2003;92:250-7.
- van Zanden JJ, Wortelboer HM, Bijlsma S, Punt A, Usta M, Bladeren PJ, et al. Quantitative structure-activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem Pharmacol 2005;69:699-708.
- Rychlik B, Pulaski L, Sokal A, Soszyñski M, Bartosz G. Transport of organic anions by multidrug resistance-associated protein in the erythrocyte. Acta Biochim Polon 2000;47:763-72.
- Rychlik B, Balcerczyk A, Klimczak A, Bartosz G. The role of multidrug resistance protein 1 (MRP1) in transport of fluorescent anions across the human erythrocyte membrane. J Membr Biol 2003;193:79-90.
- Bobrowska-Hagerstrand M, Wrobel A, Rychlik B, Bartosz G, Soderstrom T, Shirataki Y, et al. Monitoring of MRP-like activity in human erythrocytes: inhibitory effect of isoflavones. Blood Cells Molec Dis 2001;27:894-900.
- Bobrowska-Hägerstrand M, Wróbel A, Mrówczyñska L, Söderström T, Hägerstrand H. Modulation of MRP1-like efflux activity in human erythrocytes caused by membrane perturbing agents. Mol Membr Biol 2003;20:255-9.
- Trompier D, Baubichon-Cortay H, Chang XB, Maitrejean M, Barron D, Riordon JR, et al. Multiple flavonoid-binding sites within multidrug resistance protein MRP1. Cell Mol Life Sci 2003;60:2164-77.
- Hooijberg JH, Broxterman HJ, Heijn M, Fles DL, Lankelma J, Pinedo HM. Modulation by (iso)flavonoids of the ATPase activity of the multidrug resistance protein. FEBS Lett 1997;413:344-8.
- Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse P-glycoprotein. Proc Natl Acad Sci USA 1998;95:9831-6.
- Wang EJ, Barecki-Roach M, Johnson WW. Elevation of P-glycoprotein function by a catechin in green tea. Biochem Biophys Res Commun 2002;297:412-8.
- Andersen OS, Finkelstein A, Katz I, Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol 1976;67:749-71.
- Okamoto F, Okabe K, Kajiya H. Genistein, a soybean isoflavone, inhibits inward rectifier K(+) channels in rat osteoclasts. Jpn J Physiol 2001;51:501-9.
- Chiang CE, Luk HN, Chen LL, Wang TM, Ding PY. Genistein inhibits the inward rectifying potassium current in guinea pig ventricular myocytes. J Biomed Sci 2002;9:321-6.
- Cermak R, Kuhn G, Wolffram S. The flavonol quercetin activates basolateral K(+) channels in rat distal colon epithelium. Br J Pharmacol 2002;135:1183-90.
- Cermak R, Follmer U, Wolffram S. Dietary flavonol quercetin induces chloride secretion in rat colon. Am J Physiol 1998;275:1166-72.
- Lee EH, Meissner G, Kim DH. Effects of quercetin on single Ca2+ release channel behavior of skeletal muscle. Biophys J 2002;82:1266-77.
- Bulteau-Pignoux L, Derand R, Metaye T, Joffre M, Becq F. Genistein modifies the activation kinetics and magnitude of phosphorylated wild-type and G551D-CFTR chloride currents. J Membr Biol 2002;188:175-82.
- Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CTFR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol 1995;268:C886-93.
- Lansdell KA, Cai Z, Kidd JF, Sheppard DN. Two mechanisms of genistein inhibition of cystic fibrosis transmembrane conductance regulator Cl- channels expressed in murine cell line. J Physiol 2000;524:317-30.
- Randak C, Auerswald EA, Assfalg-Machleidt I, Reenstra WW, Machleidt W. Inhibition of ATPase, GTPase and adenylate kinase activities of the second nucleotide-binding fold of the cystic fibrosis transmembrane conductance regulator by genistein. Biochem J 1999;340:227-35.