Regulation of intracellular Ca2+ and calcineurin by NO/PKG in proliferation of vascular smooth muscle cells
Introduction
Injuries to vascular endothelium induce the migration, hypertrophy, and proliferation of vascular smooth muscle cells (VSMC)[1]. Compelling evidence indicates that nitric oxide (NO) negatively regulates the proliferation of VSMC via the pathways of guanosine-3',5'-cyclic monophosphate (cGMP) and cGMP-dependent protein kinase (PKG). Ca2+/calcineurin is involved in smooth muscle-specific transcrip-tion, and may be a potential target of smooth muscle cell proliferation[2–4]. However, the mechanisms by which NO/PKG inhibits VSMC proliferation remain unknown.
Therefore, it is proposed that NO/PKG can inhibit VSMC proliferation via modulation of Ca2+/calcineurin. NO donor SNAP inhibited the intracellular Ca2+ response of SMC to acetylcholine (ACh). Caffeine[5] and cGMP can modulate Ca2+ spark activity[6]. PKG regulates intracellular Ca2+ variations at multiple levels[7]. Inhibition of nuclear factors of activated T cells (NFAT) by NO-cGMP-PKG I is responsible for cardiac hypertrophy after α1-adrenoceptor stimulation[8]. However, whether or not NO/PKG inhibits the proliferation of VSMC via the modulation of Ca2+/calcineurin is unclear. In the present study, we used VSMC from rat aorta and studied the activities of NO/PKG, intracellular Ca2+, and calcineurin in VSMC proliferation.
Materials and methods
Materials NO donor (±)-S-nitroso-N-acetylpenicillamine (SNAP), phenylephrine (PE), cyclosporin A (CsA), and verapamil (Ver) were purchased from Sigma-Aldrich Corp. (St Louis, MO, USA). The PKG-selective cGMP analog Sp8-pCPT-cGMPS and PKG antagonist Rp-8-[(4-chlorophenyl)thio]-guanosine-3',5'-cyclic monophosphoro-thioate (Rp-8-pCPT-cGMPS) were from BioMol Company (Plymouth Meeting, PA, USA). Fluo-3/AM ester was obtained from Biotium Inc (Hayward, CA, USA). Rabbit anti-calcineurin Aα affinity-purified polyclonal antibody was from Chemicon Incorporated (Temecula, CA, USA). The biotinylated protein ladder detection pack was from Cell Signaling Techno-logy, Inc (Beverly, MA, USA). The calcineurin assay kit was from Nanjing Jiancheng Bioengineering Institute (NJBI) (Nanjing, China). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco/Life Technologies (Grand Island, NY, USA).
Culture of VSMC The medial layer of the thoracic aorta from 7-day-old Wistar rats was explanted and cultured in DMEM containing 5% FBS at 37 °C in 5% CO2 atmosphere. The cells were spread onto six-well plates or 35-mm dishes and cultured to a near confluent condition. Primary VSMC (<4 passage) were used.
Intracellular Ca2+ by Fluo-3/AM staining The experiment was carried out at room temperature in a darkroom. Intracellular Ca2+ concentration in VSMC was monitored using the fluorescent Ca2+ indicator, Fluo-3/AM. In brief, cells growing in a special 35-mm culture dish were loaded with Fluo-3/AM 10 µmol/L (acetoxymethyl esters) in Krebs-Ringer solution (in mmol/L: NaCl 140, KCl 5, MgCl2 0.5, HEPES 5.5, glucose 10, CaCl2 1.2, pH 7.4) containing 0.05% pluronic acid at 37 ºC for 60 min. After being washed twice with Krebs-Ringer solution to remove unhydrolyzed indicator, the dish was transferred to a chamber to which the drugs were added. Fluorescence in cells was measured using a confocal microscope. The excitation wavelength was 488 nm, and the emission wavelength was 522 nm. Change in fluorescence was expressed as F/F0, where F represents the fluorescence intensity (F) of each pixel in the original fluorescence image and F0 is defined as the intensity at the beginning of the images when the cell was assumed to be in the resting state.
Calcineurin protein expression Protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes, and the membranes were incubated with rabbit anti-calcineurin Aα affinity-purified polyclonal antibody directed against the major calcineurin catalytic subunit, calcineurin A (CnA)-α. Additional procedures followed the methods of Hammes et al[9]. The blotted antibody was visualized using chemiluminescence, and a densitometric scanner determined the density of the band.
Calcineurin enzymatic activity The activity of calcine-urin was determined using a calcineurin activity assay kit as described in the manufacturer’s protocol. The RII-phosphopeptide (BioMol) was used as a highly specific substrate for calcineurin. The detection of free inorganic phosphate released from RII by calcineurin was based on the malachite green dye reaction. Reactions were terminated after 30 min, and absorption was read on an ultraviolet spectroscope at 660 nm. The activity was corrected for protein concentration. Calcineurin activity was expressed as a percentage compared with the control group.
Cell proliferation assay After VSMC were incubated at 37 °C for 48 h, stock [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (MTT) solution was added and incubated with the cells for 4 h. The medium was then removed as completely as possible without disturbing the formazan crystals that had formed within the cells. After the addition of Me2SO (Merck, Darmstadt, Germany) into the wells the plate was shaken for a short time, and optical density was measured at 570 nm.
Cell viability VSMC were cultured to 1×104 cells/well in six-well dishes and then incubated in the absence (control) or presence of PE 10 µmol/L, SNAP 250 µmol/L, Sp-8-pCPT-cGMPS 500 µmol/L, and Rp-8-pCPT-cGMPS 100 µmol/L, or various combinations for 48 h. At the end of the incubation, nucleic acid-binding fluorescent dyes, acridine orange and ethidium bromide (10 mg/L each per well), were added. Using fluorescent microscopy, viable cells with green fluorescent nuclei and non-viable cells with red or orange fluorescent nuclei were counted, and at least 200 cells were counted for each sample. Cell viability (%)=100×(number of viable cells)/(number of cells counted).
Statistical analysis Data were expressed as mean±SEM and statistical analysis were carried out using ANOVA followed by Bonferroni or Dunn post-hoc tests. P<0.05 was considered significant.
Results
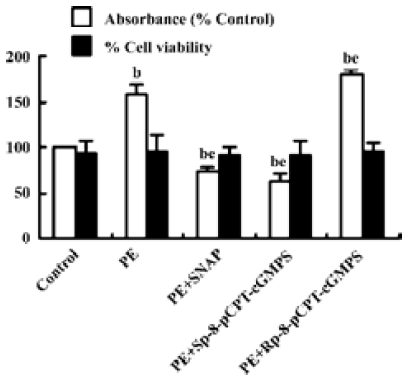
Effect of NO/PKG on the proliferation and viability of VSMC SNAP (250 µmol/L) and Sp-8-pCPT-cGMPS (500 µmol/L) decreased PE 10 µmol/L-induced proliferation of VSMC by 27.3% and 36.6%, respectively, and Rp-8-pCPT-cGMPS (100 µmol/L) increased PE-induced proliferation of VSMC. However, SNAP, Sp-8-pCPT-cGMPS, and Rp-8-pCPT-cGMPS did not affect the viability of VSMC compared with the control group (Figure 1).
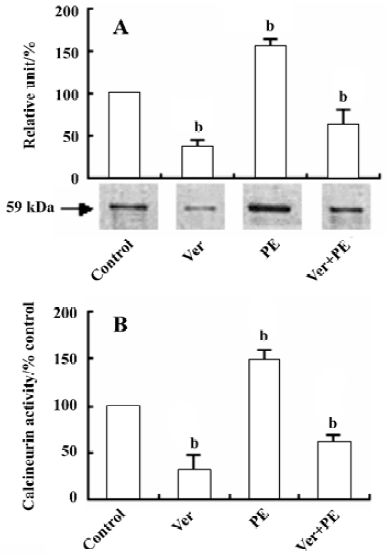
Effect of Ver on calcineurin protein expression and activity in VSMC After VSMC were pretreated with Ver (8 µmol/L) for 24 h and then incubated with PE (10 µmol/L) for another 24 h, calcineurin protein expression and its activity were decreased by 63.1% (Figure 2A) and 59.7% (Figure 2B) compared with the control group, respectively.
Effect of Ver and CsA on the downregulation of VSMC proliferation by NO/ PKG VSMC were pretreated with Ver (8 µmol/L) or CsA (500 μg/L) for 24 h to inhibit Ca2+ influx or calcineurin activity, respectively. The VSMC were then treated with SNAP (250 µmol/L), Sp-8-pCPT-cGMPS (500 µmol/L), or Rp-8-pCPT-cGMPS (100 µmol/L) for 12 h. Finally they were incubated with PE (10 µmol/L) for 12 h.
Pretreatment with Ver decreased PE-induced VSMC proliferation by 22.0% compared with the control group. Additional treatment with SNAP and Sp-8-pCPT-cGMPS further inhibited VSMC proliferation compared with the Ver pretreatment group (P<0.05, Figure 3A).
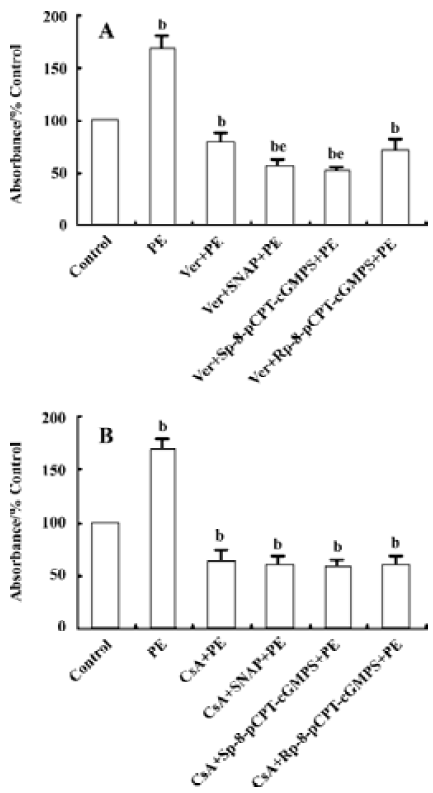
Pretreatment with CsA decreased PE-induced VSMC proliferation by 36.7% compared with the control group. Additional treatment with SNAP, Sp-8-pCPT-cGMPS, or Rp-8-pCPT-cGMPS did not further affect VSMC proliferation compared with the CsA pretreatment group (P>0.05, Figure 3B).
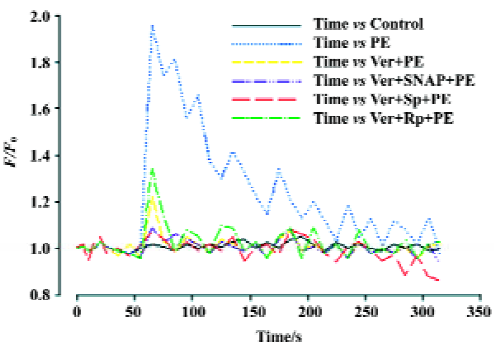
Effects of Ver and NO/PKG on variations in intracellular Ca2+ induced by PE in VSMC VSMC were pretreated with Ver (8 µmol/L) for 30 min and then incubated with SNAP (250 µmol/L), Sp-8-pCPT-cGMPS (500 µmol/L), or Rp-8-pCPT-cGMPS (100 µmol/L) for 30 min. Finally the VSMC were stimulated with PE (10 µmol/L). Intracellular Ca2+variation was inhibited by Ver. Additional treatment with SNAP and Sp-8-pCPT-cGMPS after Ver pretreatment further inhibited intracellular Ca2+ variation, but additional treatment with Rp-8-pCPT-cGMPS did not (Figure 4).
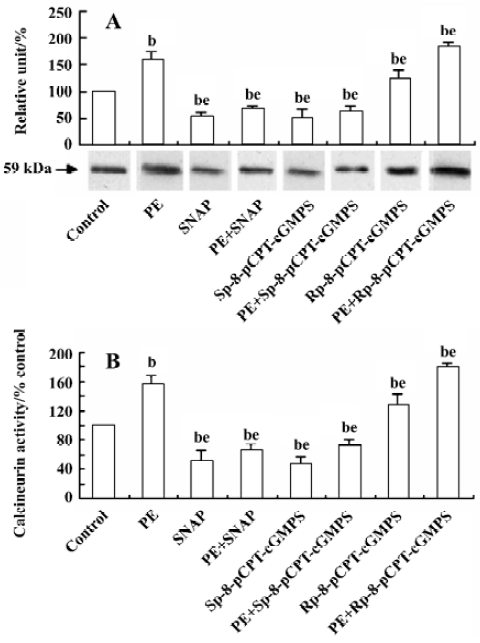
Effect of NO/PKG on calcineurin expression and activity in VSMC The increase in calcineurin protein expression and its activity induced by PE (10 µmol/L) for 24 h were inhibited by a 24-h pretreatment with SNAP (250 µmol/L) or Sp-8-pCPT-cGMPS (500 µmol/L), and was slightly promoted by a 24-h pretreatment with Rp-8-pCPT-cGMPS (100 µmol/L) (Figure 5).
Discussion
NO inhibits the proliferation of VSMC via the pathways of cGMP and PKG. NO/PKG modulates a large variety of physiological functions including vascular tone, platelet aggregation, apoptosis, and proliferation. However, the mechanisms by which NO/PKG inhibits VSMC proliferation are still unclear. The present study investigated the activities of NO/PKG, intracellular Ca2+, and calcineurin in the proliferation of VSMC.
PE, a stimulator for Ca2+ oscillations and cell growth[10,11], is used to induce intracellular Ca2+ variations and the proliferation of VSMC. Our results show that the addition of SNAP and Sp-8-pCPT-cGMPS decreases cell proliferation in cells pre-stimulated with PE by 27.3% and 36.6%, respectively, whereas the addition of Rp-8-pCPT-cGMPS increases cell proliferation. No significant changes in VSMC viability were found between the experimental groups. These results suggest that NO/PKG is involved in the inhibitory effects on SMC proliferation, but has no marked effects on SMC viability.
Ca2+ is an essential regulator of the cell cycle. The Ca2+ response control gene was expressed in various cell types[12]. In VSMC, when intracellular [Ca2+]i level increases, calcineurin is activated. Its de-phosphorylated transcription factors, NFAT, in turn promote nuclear translocation of NFAT. The NFAT transcription factors then cooperate with nuclear transcription factors and stimulate the transcriptional activation of various genes that are involved in VSMC proliferation[13]. Our results confirm that intracellular Ca2+ variations play an important role in regulating VSMC proliferation.
Recent studies have shown that NO/PKG can regulate intracellular Ca2+ variations. NO reduces the intracellular Ca2+ concentration in SMC by inhibiting sarcoplasmic reticulum (SR). Ca2+ release through both IP3R and RyR[5], and Ca2+ influx through N-channel gating via cGMP and PKG[14]. cGMP can modulate Ca2+ spark activity by decreasing myofibrillar Ca2+ sensitivity and increasing Ca2+ uptake by the SR[6]. PKG has been proposed to regulate [Ca2+]i variations in different cell types by different mechanisms[7]. For example, PKG can inhibit intracellular Ca2+ release from the endoplasmic reticulum by inhibition of IP3 formation[15] and Ca2+ entry through plasma membrane Ca2+ channels[16,17] or stimulate its efflux across the membrane by activation of a Na+/Ca2+ exchanger[18]. Bonnevier and Arner[19] reported that signals downstream of cGMP/PKG could reverse PKC-mediated Ca2+ sensitization in smooth muscle. However, whether or not NO/PKG inhibits VSMC proliferation via the regulation of intracellular Ca2+ movement has not been revealed. We found that Ver inhibited PE-stimulated intracellular Ca2+ variations, which could be further inhibited by SNAP and Sp-8-pCPT-cGMPS. These results suggest that NO/PKG can regulate PE-induced intracellular Ca2+ variations in VSMC, which is possibly achieved via regulation of Ca2+ release, Ca2+ efflux, and Ca2+ influx by NO/PKG. The definite mechanisms by which NO/PKG modulates intracellular Ca2+ variations of SMC will be studied in the future. For these reasons, we conclude that NO/PKG inhibits VSMC proliferation via modulation of intracellular Ca2+ variations.
Calcineurin is a heterodimer consisting of a 59-kDa subunit, CnA, and a 19-kDa subunit with calcineurin B (CnB) tightly bound to CnA. CnA consists of a catalytic and a regulatory domain. The regulatory domain contains the CnB binding domain, the calmodulin binding domain, and an autoinhibitory domain at the C-terminus. Ca2+ binds to both calmodulin and CnB displacing the inhibitory C-terminal peptide from the active site of CnA, thus activating phosphatase function[20]. So CnB acts as a sensor for changes in intracellular Ca2+. Calcineurin is a downstream target of intracellular Ca2+. Increase in intracellular Ca2+ concentration will activate calcineurin, thus inducing proliferation-related gene transcription. Our results further demonstrate that intracellular Ca2+ variation plays an important role in regulating the expression and activity of calcineurin in VSMC, and that calcineurin is a potential target for treatment of diseases related to SMC proliferation. However, it remains to be determined whether NO/PKG decreases the proliferation of VSMC via calcineurin. Our study shows that the pre-addition of CsA decreases PE-induced proliferation by 36.7% compared with controls. CsA had no influence on the inhibitory effects of SNAP, Sp-8-pCPT-cGMP, and Rp-8-pCPT-cGMPS. These results suggest that NO/PKG inhibits VSMC proliferation via calcineurin. Furthermore, our study demonstrates that SNAP and Sp-8-pCPT-cGMPS reduced, but Rp-8-pCPT-cGMPS increased, calcineurin protein expression and its activity in SMC stimulated by PE. Therefore, we conclude that NO/PKG inhibits VSMC proliferation by regulating calcineurin expression and its activity.
In addition, Sp-8-pCPT-cGMPS has the ability to stimulate both PKG and cAMP-dependent protein kinase (PKA) with similar potency. cAMP/PKA is able to induce Ca2+ desensitization by inhibition of the muscarinic receptor signaling upstream from Rho activation and preferentially reverse PKC-mediated Ca2+ sensitization in SMC[21]. It is possible that PKA is partially involved in the regulation of calcineurin by changing intracellular Ca2+ variations in SMC proliferation.
In conclusion, NO/PKG partially inhibits the proliferation of VSMC without affecting their viability. It is associated with the regulation of calcineurin activity by modulating intracellular Ca2+ concentration.
Acknowledgements
We thank Prof Chao-shu TANG, Prof Xian WANG, Prof Yuan-sheng GAO, Prof Ding-feng SU, and Prof Ding-fang BU for their kind help in preparing this manuscript.
References
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999;340:115-26.
- Chen Y, Zhao M, Xia C. Effect of urotensin II on the airway smooth muscle cell proliferation and its mechanism. Chin J Med 2000;80:928-30.
- Wada H, Hasegawa K, Morimoto T, Kakita T, Yanazume T, Abe M, et al. Calcineurin-GATA-6 pathway is involved in smooth muscle-specific transcription. J Cell Biol 2002;156:983-91.
- Lipskaia L, Pourci ML, Delomenie C, Combettes L, Goudouneche D, Paul JL, et al. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res 2003;92:1115-22.
- Kannan MS, Prakash YS, Johnson DE, Sieck GC. Nitric oxide inhibits calcium release from sarcoplasmic reticulum of porcine tracheal smooth muscle cells. Am J Physiol 1997;272:L1-7.
- Satoh S, Makino N. Intracellular mechanisms of cGMP-mediated regulation of myocardial contraction. Basic Res Cardiol 2001;96:652-8.
- Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci 2000;113:1671-6.
- Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA 2002;99:11363-8.
- Hammes A, Oberdorf-Maass S, Rother T, Nething K, Gollnick F, Linz KW, et al. Overexpression of the sarcolemmal calcium pump in the myocardium of transgenic rats. Circ Res 1998;83:877-88.
- Hamada H, Damron DS, Hong SJ, Van Wagoner DR, Murray PA. Phenylephrine-induced Ca2+ oscillations in canine pulmonary artery smooth muscle cells. Circ Res 1997;81:812-23.
- Shaw L, O'Neill S, Jones CJ, Austin C, Taggart MJ. Comparison of U46619-, endothelin-1- or phenylephrine-induced changes in cellular Ca2+ profiles and Ca2+ sensitisation of constriction of pre-ssurised rat resistance arteries. Br J Pharmacol 2004;141:678-88.
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 1997;386:855-8.
- Suzuki E, Nishimatsu H, Satonaka H, Walsh K, Goto A, Omata M, et al. Angiotensin II induces myocyte enhancer factor 2- and calcineurin/nuclear factor of activated T cell-dependent transcriptional activation in vascular myocytes. Circ Res 2002;90:1004-11.
- D’Ascenzo M, Martinotti G, Azzena GB, Grassi C. cGMP/protein kinase G-dependent inhibition of N-type Ca2+ channels induced by nitric oxide in human neuroblastoma IMR32 cells. J Neurosci 2002;22:7485-92.
- Furuichi T, Mikoshiba K. Inositol 1, 4, 5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J Neurochem 1995;64:953-60.
- Mery PF, Lohmann SM, Walter U, Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci USA 1991;88:1197-201.
- Clapp LH, Gurney AM. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflügers Arch 1991;418:462-70.
- Furukawa KI, Ohshima N, Tawada-Iwata Y, Shigekawa M. Cyclic GMP stimulates Na+/Ca2+ exchange in vascular smooth muscle cells in primary culture. J Biol Chem 1991;266:12337-41.
- Bonnevier J, Arner A. Actions downstream of cyclic GMP/protein kinase G can reverse protein kinase C-mediated phosphorylation of CPI-17 and Ca2+ sensitization in smooth muscle. J Biol Chem 2004;279:28998-9003.
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 1998;273:13367-70.
- Endou K, Iizuka K, Yoshii A, Tsukagoshi H, Ishizuka T, Dobashi K, et al. 8-Bromo-cAMP decreases the Ca2+ sensitivity of airway smooth muscle contraction through a mechanism distinct from inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 2004;287:L641-8.
