Signal pathways underlying homocysteine-induced production of MCP-1 and IL-8 in cultured human whole blood1
Introduction
Hyperhomocysteinemia is an independent risk factor for atherosclerosis and venous thrombosis. We have found that an increased homocysteine (Hcy) levels in cultured whole blood can promote the production of monocyte chemo-attractant protein-1 (MCP-1) and interleukin-8 (IL-8)[1]. As potent proatherosclerotic factors, MCP-1 and IL-8 are considered to be vital contributing factors in the pathogenesis of atherosclerosis and venous thrombosis[2–6]. Therefore, increased MCP-1 and IL-8 levels in plasma may be an important mechanism by which hyperhomocysteinemia promotes the progression of atherosclerosis.
In addition, the relationship between hyperhomocy-steinemia and the cardiovascular disease and thrombosis has been well established. Recent clinical investigations have found that hyperhomocysteinemia is also associated with many different diseases such as inflammation, autoimmune diseases, and cognitive diseases[7–13]. These new findings imply that Hcy may also mediate the development of these medical conditions by presently-unknown mechanism(s).
Previous studies have found that the signaling pathways involving protein kinase C (PKC), protein tyrosine kinase (PTK), or mitogen-activated protein kinase (MAPK) play a vital role in the mediation of MCP-1 and IL-8 production in response to other stimuli[14–18]. Thus, these signaling pathways are examined in the present study to explore which signaling pathways are involved in the Hcy-induced production of MCP-1 and IL-8. Our results presented here demonstrate that inhibitors of PKC, CaM, MAPK and NF-κB inhibit Hcy-induced MCP-1 and IL-8 production in cultured whole blood. Our results suggest that activation of these signaling pathways is involved in Hcy-induced chemokine production. Considering the fact that abnormally activated PKC, CaM, MAPK, and NF-κB play important roles in the initiation and progression of autoimmune and cognitive diseases[19-22], our study provides useful clues as to why Hcy may be involved in these diseases.
Materials and methods
Human whole blood culture The investigation conforms to the principles outlined in the Declaration of Helsinki. The human whole blood cultures were slightly modified from our previous studies[23,24]. Briefly, blood from healthy donors was drawn into heparinized syringes. Whole blood was then placed on a rotator and incubated at 37 ºC in an atmosphere containing 5% CO2. Cell viability was evaluated by Trypan blue exclusion. Only cell preparations with a 95% or greater viability were used.
Measurement of MCP-1 and IL-8 protein secretion Human whole blood was treated with Hcy for indicated times and/or preincubated for 60 min with genistein, tyrphostin, calphostin C, RO-31-8220, W7, SB 203580, PD 98059, and curcumin or other pharmacological reagents. The plasma was harvested and transferred to other polypropylene tubes and stored at -30 ºC for not more than 1 week before measurement of chemokines MCP-1 and IL-8 protein concentrations in the plasma, which were determined by ELISA (R&D Systems Inc, Minneapolis, MN).
Chemicals L-homocysteine (L–Hcy), L-cysteine, L-methione, genistein, tyrphostin, and pyrrolidine dithiocarbamate (PDTC) were purchased from Sigma Co (St Louis, MO). Calphostin C, RO-31-8220, W7, SB 203580, PD 98059, and curcumin were purchased from Calbiochem Co (La Jolla, CA). 2´-7´-DCFH-DA was obtained from Molecular Probes (Eugene, OR). RPMI-1640 was purchased from Gibco Laboratories (Grand Island, NY). Other chemicals were purchased from the Chinese Chemical Co (Beijing, China).
Statistical analysis Results are expressed as mean±SEM. The number of samples used for each group is presented in the figure legends. The data were analyzed using one-way ANOVA and further analyzed using the Student-Newmen-Keuls test for multiple comparisons within treatment groups or the t-test (unpaired test with Welch’s correction) for comparison between two groups with non-normal distribution. P<0.05 was considered a significant difference between treatment groups.
Results
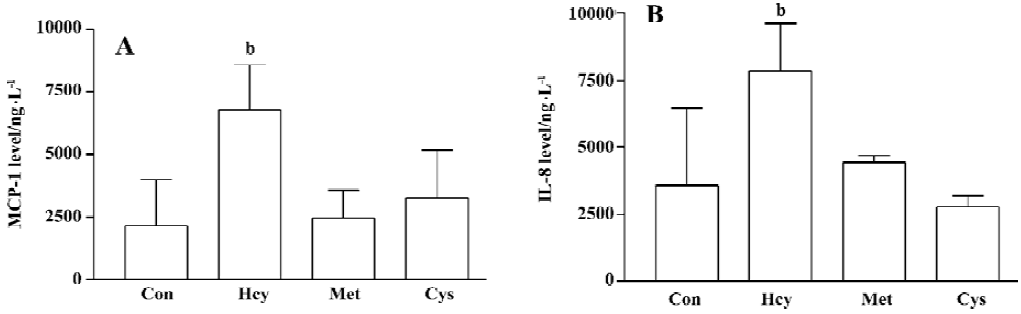
Effect of L-methionine and L-cysteine on MCP-1 and IL-8 production from human whole blood To understand the role of thiol residues in Hcy-induced chemokine production, human whole blood was treated with sulfur-containing amino acids, L-methionine and L-cysteine 100 µmol/L for 32 h. Neither L-methionine nor L-cysteine elevated the production of MCP-1 and IL-8 in cultured human whole blood (Figure 1A, 1B). These data suggest that the thiol residues do not play an important role in the Hcy mechanisms.
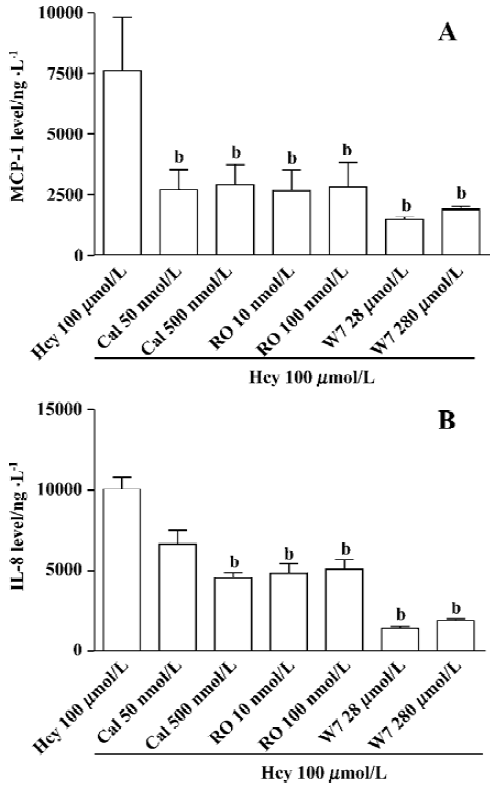
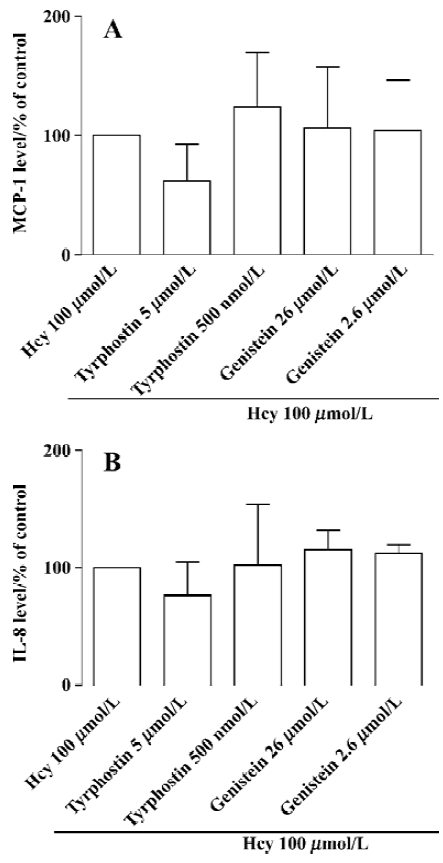
PKC, PTK, and CaM in Hcy-induced secretion of MCP-1 and IL-8 A substantial body of evidence indicates that activation of PKC, CaM, PTK, MAPK, and NF-κB may be involved in chemokine production. Therefore, we hypothesize that such signaling pathways might contribute to Hcy-induced chemokine expression and secretion in cultured whole blood. Human whole blood was pretreated with inhibitors of CaM (W7, 28–280 µmol/L), PKC (calphostin C, 50–500 nmol/L and RO-31-8220, 10–100 nmol/L), PTK (genistein, 2.6-26 µmol/L and tyrphostin, 0.5-5 µmol/L). CaM and PKC inhibitors significantly inhibited Hcy-induced MCP-1 and IL-8 production in cultured human whole blood (Figure 2). However, the inhibitors of PTK had no obvious effect on chemokine production (Figure 3). These data show that the activated signaling pathways of PKC and CaM are involved in the Hcy-induced production of MCP-1 and IL-8 in cultured whole blood.
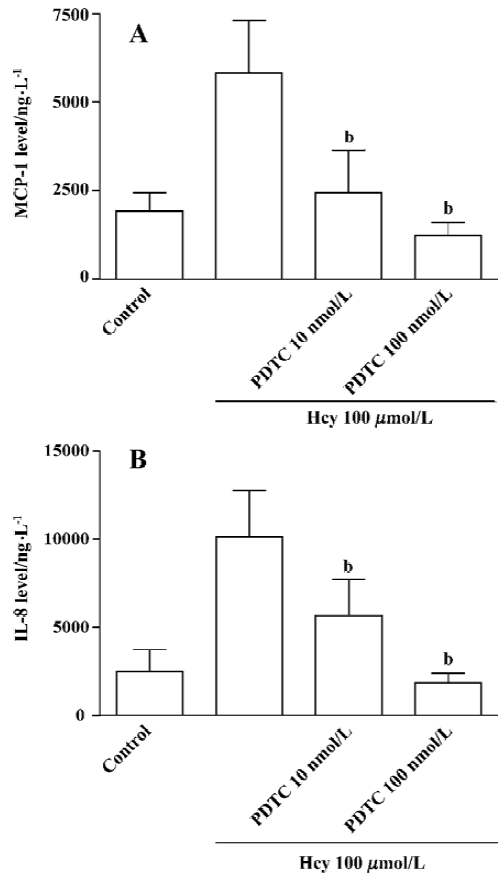
MAPK and NF-κB in Hcy-induced MCP-1 and IL-8 production To further study the role of the downstream signaling molecules of PKC and CaM, such as MAPK and NF-κB, in the Hcy-induced MCP-1 and IL-8 production, the inhibitors of ERK1/2 MAPK (PD 98059, 2–20 µmol/L), p38 MAPK (SB 203580, 0.6-6 µmol/L), JNK MAPK (curcumin, 2–10 µmol/L), and NF-κB (PDTC, 10–100 nmol/L) were added to cultured whole blood for 60 min, respectively. This was followed by stimulation with Hcy 100 µmol/L for 32 h. As shown in Figure 4, MAPK (p38, ERK1/2, and JNK) inhibitors significantly inhibited Hcy-induced MCP-1 and IL-8 production in cultured human whole blood. In addition, the NF-κB inhibitor PDTC also prevented Hcy-induced MCP-1 and IL-8 production in the cultured whole blood (Figure 5A, 5B). These data show that the activation pathways of MAPK and NF-κB are involved in the regulation of the Hcy-induced secretion of MCP-1 and IL-8. In the range of inhibitors examined, Hcy (10–1000 µmol/L) did not significantly increase LDH release as compared with the control (data not shown), indicating that Hcy plus the inhibitors did not have an obvious toxic effect on human whole blood.

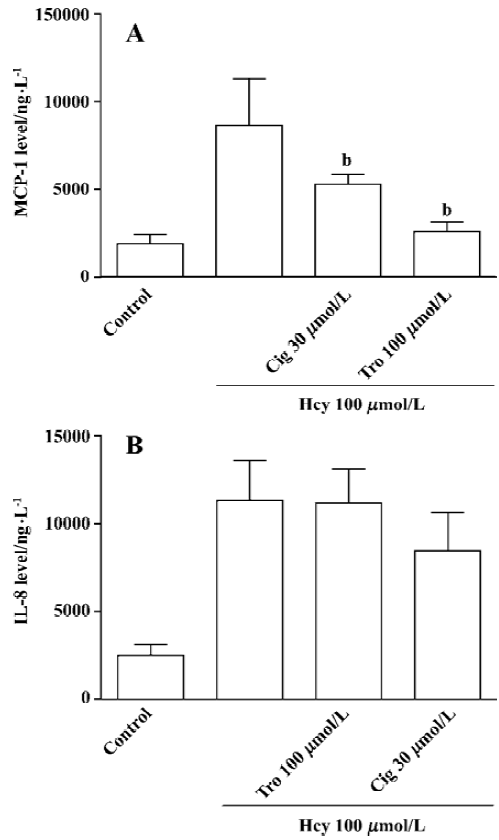
Influence of PPARγ activators on Hcy-induced MCP-1 and IL-8 production Cultured whole blood was pretreated with the activators of PPARγ (ciglitazone 30 µmol/L and troglitazone 10 µmol/L) for 60 min, respectively. It is followed by stimulation with Hcy 100 µmol/L for 32 h. The data showed that PPARγ activators depressed the Hcy-induced MCP-1 production but not IL-8 production in the cultured whole blood (Figure 6A, 6B).
Discussion
Our previous work showed that an increased Hcy level promoted the production of MCP-1 and IL-8 in cultured whole blood. This suggests that hyperhomocysteinemia may upregulate MCP-1 and IL-8 levels in plasma and consequently promote the initiation and progression of atherosclerosis and venous thrombosis. Our present study demonstrated that activated signaling pathways such as PKC, CaM, MAPK, and NF-κB were involved in the mediation of Hcy-induced MCP-1 and IL-8 production. These data indicate that Hcy promotes MCP-1 and IL-8 production in cultured whole blood by activating these signaling pathways. It further suggested that abnormally activated signaling pathways, which were caused by Hcy, might play roles as vital mechanisms underlying Hcy-mediated inflammatory, autoimmune, and cognitive diseases.
PKC is thought to play an important role in Hcy-induced MCP-1 production in cultured vascular smooth muscle cell lines, but not in endothelial lines[25,26]. Our present results shows that the activation of PKC is necessary for both IL-8 and MCP-1 production induced by Hcy, since calphostin C and RO31-8220, inhibitors of PKC, significantly reduced MCP-1 and IL-8 synthesis. No inhibitory effect was observed with the PTK inhibitors, genistein and tyrphostin, which indicates that protein tyrosine kinase might not be involved in Hcy-mediated MCP-1 and IL-8 synthesis. Previous studies have also shown that calcium/CaM plays an important role in the mediation of IL-8 production in several other cell systems[27,28]. To test whether calcium/CaM is involved in the Hcy-induced chemokine synthesis, we used W7, a potent inhibitor of CaM, to study its influence on Hcy-induced chemokine synthesis in cultured whole blood. Our data demonstrated that W7 significantly decreased both MCP-1 and IL-8 production. Taken together, these results reveal that both activated PKC and CaM are involved in Hcy-induced MCP-1 and IL-8 production in cultured whole blood. PTK, however, had no significant effect on Hcy action.
MAPKs represent a family of eukaryotic protein kinases involved in various cellular processes. Three parallel cascades are now commonly described, each of which is named after its end-moiety: p38, the extracellular signal regulated protein kinases (ERK), and stress activated protein kinase/c-Jun N-terminal kinases (JNK)[29]. As the upstream signaling molecules of MAPK, the activated PKC and CaM may mediate the activation of MAPK, such as MEK, p38MAPK, and JNK. For example, as the intermediate signaling pathway molecules, MAPK is indispensable in the PKC-mediated signaling transduction system[30,31]. Thus, we investigated specific downstream signaling molecules that could be potentially important as the targets of activated PKC and CaM. We demonstrated that PD98059, SB 203580, and curcumin, the selective inhibitors of MEK1, p38 MAPK, and JNK respectively, significantly decreased IL-8 and MCP-1 production. This data is consistent with other reports that MAPK is required for IL-8 and MCP-1 production in several other cell systems in response to various stimuli[30].
NF-κB is an important transcription factor in the initiation of cell growth and secretion. Consistent with its role as a primer in synthesis, NF-κB binds to the IκB site of various gene promoter regions[32]. Our data demonstrate that Hcy-induced chemokine production is almost competently blocked by PDTC, a specific inhibitor of NF-κB. These results agree with those obtained in other studies[33], suggesting that NF-κB has a role in the regulation of IL-8 and MCP-1 production.
Another interesting finding is that PPAR-γ activation can lead to a decrease in Hcy-induced MCP-1 production in cultured whole blood. PPAR-γ is a ligand-activated transcription factor belonging to the nuclear receptor family. PPAR-γ is expressed in differentiated human mono/macrophages and functions as a regulator of cellular proliferation, differen-tiation, and apoptosis[34]. Although PPAR-γ seems to be absent from isolated monocytes, PPAR-γ can regulate mono/macrophage physiology[34,35]. Furthermore, Jiang et al[36] reported that incubation of human monocytes with the natural PPAR-γ ligand, or with synthetic agonists, inhibited the production of proinflammtory cytokines. Other studies showed that PPAR-γ inhibited the transcriptional activity of genes by interfering with transcription factors such as NF-κB[37]. Our data showed that Hcy-mediated MCP-1 and IL-8 production was NF-κB-dependent, thereby the influence of activated PPAR-γ in the Hcy-induced chemokine production was detected. The activators of PPAR-γ significantly depressed the production of MCP-1 but not IL-8. It suggests that this is an important pathway for attenuating the damage of Hcy or other inflammatory mediators. The different inhibitory effects on MCP-1 and IL-8 production imply that the regulation of Hcy-induced MCP-1 and IL-8 production is slightly different, at least in the cultured whole blood system.
Our previous studies and other work showed that Hcy potentiated lymphocyte proliferation. Also the thiol-containing compounds, such as cysteine had similar effects on lymphocyte proliferation[17,18]. The other compounds without thiol have no such effect. This suggests that thiol residue plays a key role in Hcy-induced lymphocyte proliferation. Our current studies found that thiol-containing compounds, such as cysteine and methionine, failed to promote MCP-1 and IL-8 production in the cultured whole blood. These results are consistent with previous work[38], suggesting that thiol may play a less important role in Hcy-induced chemo-kine production. Thus, there is the possibility that different sites of Hcy are responsible for different Hcy action.
Our current research has three implications; first, Hcy-induced MCP-1 and IL-8 production is mediated by activated signaling pathways such as PKC, CaM, MAPK, and NF-κB. Second, although the exact mechanism is unclear, current studies suggest that Hcy is able to activate the PKC, CaM, MAPK, and NF-κB signaling pathways. When we take the consideration that these activated signaling pathways are involved in many pathological and physiological functions, especially, in the initiation and progression of many immune and cognitive diseases, our findings shed light on a possible answer to why hyperhomocysteinemia has been found to be linked with not only cardiovascular diseases, but also so many inflammatory, autoimmune and cognitive diseases. Finally, activated PPAR-γ inhibited Hcy-induced MCP-1 production. This study provides a novel approach to partly attenuate the proatherogenic effect of hyperhomo-cysteinemia.
Footnote
Project supported by the Major National Basic Research Program of China (N
References
- Zeng X, Remick DG, Wang X. Homocysteine induces production of monocyte chemoattractant protein-1 and interleukin-8 in cultured human whole blood. Acta Pharmacol Sin 2004;25:1419-25.
- Reape TJ, Groot P. Chemokines and atherosclerosis. Atherosclerosis 1999;147:213-5.
- de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 2003;107:690-5.
- Romuk E, Skrzep-Poloczek B, Wojciechowska C, Tomasik A, Birkner E, Wodniecki J, et al. Selectin-P and interleukin-8 plasma levels in coronary heart disease patients. Eur J Clin Invest 2002;32:657-61.
- Van Aken BE, den Heijer M, Bos GM, van Deventer SJ, Reitsma PH. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost 2000;83:536-9.
- Van Aken BE, Reitsma PH, Rosendaal FR. Interleukin 8 and venous thrombosis: evidence for a role of inflammation in thrombosis. Br J Haematol 2002;116:173-7.
- Morgenstern I, Raijmakers MT, Peters WH, Hoensch H, Kirch W. Homocysteine, cysteine, and glutathione in human colonic mucosa: elevated levels of homocysteine in patients with inflammatory bowel disease. Dig Dis Sci 2003;48:2083-90.
- Yxfeldt A, Wallberg-Jonsson S, Hultdin J, Rantapaa-Dahlqvist S. Homocysteine in patients with rheumatoid arthritis in relation to inflammation and B-vitamin treatment. Scand J Rheumatol 2003;32:205-10.
- Vrethem M, Mattsson E, Hebelka H, Leerbeck K, Osterberg A, Landtblom AM, et al. Increased plasma homocysteine levels without signs of vitamin B12 deficiency in patients with multiple sclerosis assessed by blood and cerebrospinal fluid homocysteine and methyl-malonic acid. Mult Scler 2003;9:239-45.
- Lazzerini PE, Capecchi PL, Bisogno S, Galeazzi M, Marcolongo R, Pasini FL. Reduction in plasma homocysteine level in patients with rheumatoid arthritis given pulsed glucocorticoid treatment. Ann Rheum Dis 2003;62:694-5.
- Martinez-Taboada VM, Bartolome MJ, Fernandez-Gonzalez MD, Blanco R, Rodriguez-Valverde V, Lopez-Hoyos M. Homocysteine levels in polymyalgia rheumatica and giant cell arteritis: influence of corticosteroid therapy. Rheumatology (Oxford) 2003;42:1055-61.
- Miller AL. The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 2003;8:7-19.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002;48:471-81.
- Rovin BH, Tan LC. Role of protein kinase pathways in IL-1-induced chemoattractant expression by human mesangial cells. Kidney Int 1994;46:1059-68.
- Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB Int J Mol Med 1999;4:223-30. (Review).
- Steube KG, Meyer C, Schupp P, Proksch P, Drexler HG. Differential effects of staurosporine and its analogues on chemokine release by promyelocytic leukemia cell line NB-4. Leuk Res 2003;27:957-63.
- Zhang Q, Zeng X, Guo J, Wang X. Effect of homocysteine on murine splenic B lymphocyte proliferation and its signal transduction mechanism. Cardiovasc Res 2001;52:328-36.
- Zhang Q, Zeng X, Guo J, Wang X. Oxidant stress mechanism of homocysteine potentiating ConA-induced proliferation in murine T lymphocytes. Cardiovasc Res 2002;53:1035-42.
- Buchs AE, Rapoport MJ. T cell signaling and autoimmune diabetes. T cell signaling and autoimmune diabetes. J Pediatr Endocrinol Metab 2000;13:1549-54.
- Marchetti C. Molecular targets of lead in brain neurotoxicity. Neurotox Res 2003;5:221-36.
- Kumar S, Blake SM, Emery JG. Intracellular signaling pathways as a target for the treatment of rheumatoid arthritis. Curr Opin Pharmacol 2001;1:307-13.
- Drosos AA. Newer immunosuppressive drugs: their potential role in rheumatoid arthritis therapy. Drugs 2002;62:891-907.
- DeForge LE, Kenney JS, Jones ML, Warren JS, Remick DG. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol 1992;148:2133-41.
- DeForge LE, Fantone JC, Kenney JS, Remick DG. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest 1992;90:2123-9.
- Sung FL, Slow YL, Wang G, Lynn EG. O K. Homocysteine stimulates the expression of monocyte chemoattractant protein-1 in endothelial cells leading to enhanced monocyte chemotaxis. Mol Cell Biochem 2001;216:121-8.
- Wang G, Siow YL. O K. Homocysteine stimulates nuclear factor kappaB activity and monocyte chemoattractant protein-1 expression in vascular smooth-muscle cells: a possible role for protein kinase C. Biochem J 2000;352:817-26.
- Miura I, Miyamoto K, Nakamura K, Watanabe Y. Hydrogen peroxide induced chemokine production in the glia-rich cultured cerebellar granule cells under acidosis. Life Sci 2002;70:821-31.
- Yu Y, De Waele C, Chadee K. Calcium-dependent interleukin-8 gene expression in T84 human colonic epithelial cells. Inflamm Res 50:220-6.
- Kim RD, Stein GS, Chari RS. Impact of cell swelling on proliferative signal transduction in the liver. J Cell Biochem 2001;83:56-69.
- Bian ZM, Elner VM, Yoshida A, Kunkel SL, Elner SG. Signaling pathways for glycated human serum albumin-induced IL-8 and MCP-1 secretion in human RPE cells. Invest Ophthalmol Vis Sci 2001;42:1660-8.
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem 1995;270:14843-6.
- Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol 2001;159:387-97.
- Zoja C, Angioletti S, Donadelli R, Zanchi C, Tomasoni S, Binda E, et al. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-kappaB dependent up-regulation of IL-8 and MCP-1. Kidney Int 2002;62:846-56.
- Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem 1998;273:25573-80.
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998;391:79-82.
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998;391:82-6.
- Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest 1999;104:383-9.
- Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells. Circulation 2001;103:2717-30.
