Construction of phospholamban antisense RNA recombinant adeno-associated virus vector and its effects in rat cardiomyocytes1
Introduction
Alterations in intracellular calcium signaling has played a key role in the pathophysiology of heart failure, and gene therapy has been identified as an important tool to help understand the relative contribution of specific calcium-handing proteins in heart failure[1].
Phospholamban (PLB) was a critical regulator of the Ca2+ affinity of the cardiac sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) and of cardiac contractility[2]. Studies on PLB-knockout and PLB-over expression mice have indicated that the expression ratio of PLB to SERCA2a was a potential target for improvement of diastolic function of the failing heart[3]. The ablation of PLB in mice resulted in stimulated SR Ca2+ uptake and enhanced contractile performance, whereas PLB over expression resulted in opposite effects[4,5]. Thus, PLB is an attractive target for treatment of cardiovascular diseases, and decreasing the level of PLB through the use of PLB antisense RNA (asPLB) would be a novel strategy for gene therapy of heart failure[6,7].
The ideal vector for myocardial gene delivery would allow the efficient and stable transduction of cardiomyocytes. The method of injection of plasmid DNA vectors directly into the left ventricular myocardium has been limited by the lower efficiency of cardiomyocyte transduction, while, the adenovirus-mediated gene transfer had been limited by immue responses to viral and foreign transgene protein. In the present study, adeno-associated viruses (AAV) were chosen as the gene transfer vector because AAV featured versatility in the host rang, long-term gene transfer potential, and minimum immune response. Recombinant adeno-associated viruses (rAAV) of rAAV-asPLB and rAAV-LacZ were constructed by AAV Helper-Free system, and the effect on expression of PLB in cultured rat cardiomyocytes was analyzed. This was to make arrangements for future PLB gene therapy in animal models.
Materials and methods
Plasmids and reagents The AAV Helper-Free System was obtained from Stratagene. The system includes: pAAV-MCS containing inverted terminal repeat (ITR) of adeno-associated virus, multiple clone site (MCS), and cytomegalovirus enhance/promotor and SV 40 poly-adenylation; pAAV-RC containing AAV replication and capsid genes; pHelper contains adenovirus-derived genes (i.e. E2A, E4, and VA RNA gene); pAAV-LacZ containing ITR and report gene lacZ. AAV-293 cells and XL10-Gold ultracompetent E coli cells, were also obtained from Stratagene, too. Restriction enzymes were obtained from Promega. Mouse monoclonal antibody and IgG were from ABR. Fluo 3-AM was from Molecular Probes, Eugene, OR, USA.
Cloning of rat PLB cDNA and construction of PLB antisense RNA plasmid vector (pAAV-asPLB) Rat PLB cDNA fragment (695 bp) was synthesized by reverse transcription-polymerase chain reaction (RT-PCR) from total RNA isolated from the heart of Wistar rats. The primers used for PCR contained a BamH I (5´) and EcoR I (3´) linker (in lower-case letters): 5´-cgggatccCGCAGCTGAGCTGA-GCTCCAGACTTCA-3´, and 5´-cggaattcTTTAAATTTCA-TTTATTCCCCAA-3´. PCR was carried out as follows: 94 ºC for 5 min followed by 30 cycles at 94 ºC for 30 s, 55 ºC for 60 s, and 72 ºC for 60 s, followed by the final step at 72 ºC for 10 min.
The synthesized PLB cDNA was subsequently digested by BamH I and EcoR I simultaneously, while the adeno-associated virus plasmid pAAV-MCS digested by corresponding restricted endonuclease enzyme. The ligation reaction was conducted with DNA ligase at 4 ºC for 16 h. The pAAV-asPLB was generated by cloning the PLB cDNA into EcoR I/BamH I sites of pAAV-MCS reversed orientation relative to the promoter. Identification of the gene was confirmed by restriction enzymes digestion and sequencing.
Construction of recombinant adeno-associated virus To construct the rAAV, AAV-293 cells were co-transfected with three AAV Helper-Free System plasmids: an ITR-containing plasmid (pAAV-asPLB or pAAV-LacZ), pAAV-RC, and pHelper. AAV-293 cells maintained in DMEM medium supplemented with 10% fetal calf serum, in a 37 ºC incubator at 5% CO2. When 293 cells reached 70%–80% confluence in 100-mm culture plate, the concentration of each plasmid was adjusted to 1 g/L in TE buffer, pH 7.5. The three plasmids (10 µL of each) and CaCl2/HBS were mixed gently, and the mixture were co-transfected into 293 cells immediately. After 6 h, the medium was replaced with 10 mL of fresh DMEM medium, and 293 cells were cultured for an additional 66–72 h.
To monitor the progress of AAV particle production, 293 cells were divided into four groups according to the difference of mixture: (1) AAV-asPLB group: pAAV-asPLB, pAAV-RC, pHelper, and CaCl2/HBS; (2)AAV- LacZ group: pAAV-LacZ, pAAV-RC, pHelper, and CaCl2/HBS, which served as a positive control; (3) pAAV-RC, pHelper, and CaCl2/HBS, which served as a negative control; (4) only CaCl2/HBS served as a blank control.
The 293 transfected cells were subject to four rounds of freeze/thaw and centrifugation at 10 000×g for 10 min. The supernatant (primary rAAV) was purified by CsCl gradient centrifugation. rAAV was verified by PCR. The genome titer and infectious titer of rAAV were measured by PCR and in site β-galactosidase staining according to instruction[8,9].
Rat cardiomyocytes culture and infection Rat neonatal cardiomyocytes were isolated, cultured conventionally, and infected with the rAAV at a multiplicity of infection (MOI) of 100 for 48 h[10]. Cardiomyocytes were divided into three groups: (1) infected with rAAV-asPLB; (2) infected with rAAV- LacZ; (3) normal control.
X-gal staining to detect the infection efficiency Cardio-myocytes were fixed with glutaraldehyde-formaldehyde solution for 10 min at room temperature, stained overnight with staining solution that contains X-gal. In infected cells, β-galactosidase cleaves X-gal to produce a blue stain. The infection efficiency was the percentage of stained cells in the total population.
Semi-quantitative RT-PCR analysis of PLB and SERCA gene expression Total cellular RNA was isolated from cells using TRIzol. Semiquantitative RT-PCR was performed to analyse PLB and SERCA gene expression. β-actin (332 bp) served as endogenous control. The primers of β-actin used for PCR were: 5´-GAGACCTTCAACACC-CCAGCC-3´, and 5´-GGCCATCTCTTGCTCGAAGTC-3´. The primers of PLB gene were described in previous text. The primers of SERCA (134 bp) gene were: 5´-AAGCAGT-TCATCCGCTACCT-3´, and 5´-AGACCATCCGTCACCA-GATT-3´. PCR products were resolved on a 2% agarose gel and the bands could be seen using ethidium bromide staining. Each band was analyzed on Kodak Digital Science Image Analysis System. The gene expression level was determined semi-quantitatively by calculating the ratio of density metric value from the PLB gene in relation to internal standards.
Western blot analysis of PLB and SERCA protein expression Cells were lysed in lysis solution at 4 ºC. The concentration of the protein in each lysate was determined with Coomassie Brilliant Blue G-250. Protein was fractionated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. The membrane was incubated with monoclonal antibody (1:1000) and IgG (1:500). The immune complexes were visualized by the ECL chemiluminescence method. Each protein band was analyzed on Kodak Digital Science Image Analysis System. The protein expression level was determined by calculating the ratio of density metric value from rAAV infected group in relation to normal control.
SERCA activity The activity of SERCA was determined following the methods of Larsen[11]. Assays for complete quantification of Ca2+-ATPase in homogenates of rat ventricular myocardium by determination of Ca2+-dependent p-nitrophenyl phosphatase (pNPPase) activities were evaluated and optimized.
Measurement of [Ca2+]i[12] After Fluo 3-AM (5 µmol/L) loading, the coverslip was mounted in the cell chamber with 200 µL Hanks’ solution. The fluorescence intensity (FI) was detected by confocal microscope (Leica, Mannheim, Germany) and inverted microscope with 40×objective and 488 nm blue laser for excitation and 530 nm for emission at room temperature. Stimulating agent (isoproterenol, 50 µmol/L) 10 µL was added to the preparation and the data were stored in disk. The fluorescence signals were normalized as ΔF/F0 (%), where F0 was the resting fluorescence and ΔF was Ca2+ change relatively the resting fluorescence.
Statistical analysis Data were expressed as mean±SD and statistically compared by one-way ANOVA with Dunnett’s test. P<0.05 was taken as statistically significant.
Results
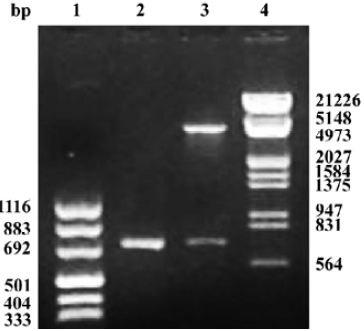
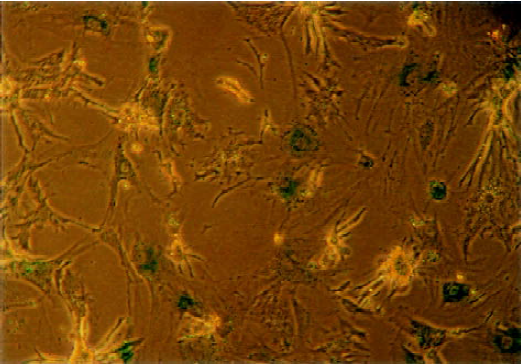
Construction and infection efficiency of recombinant adeno-associated virus The results of pAAV-asPLB restriction enzymes digestion and sequencing demonstrated that the sequence and direction of PLB cDNA fragment (695 bp) were precise (Figure 1). The rAAV-asPLB and rAAV-LacZ were harvested in rAAV-asPLB group and rAAV-LacZ group, respectively. However, the negative control and blank control showed nothing. The genome titer and infectious titer of rAAV were 1×1015 particles/L and 3×1013 infectious units/L, respectively. Cardiomyocytes were infected with rAAV-LacZ and stained with X-gal, the percentage of blue stained cells is approximately 80% (Figure 2).
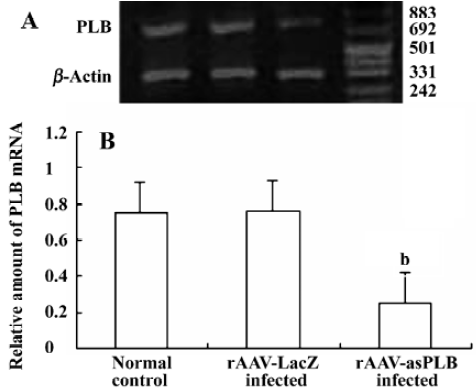
PLB mRNA expression in infected cardiomyocytes Total RNA was extracted from rAAV-asPLB infected cardiomyocytes, rAAV-LacZ infected cardiomyocytes, and normal control cardiomyocytes, respectively. The result of RT-PCR showed that the level of PLB mRNA expression was decreased in rAAV-asPLB infected cardiomyocytes compared with rAAV-LacZ infected cardiomyocytes and normal control cardiomyocytes (P<0.05, Figure 3).
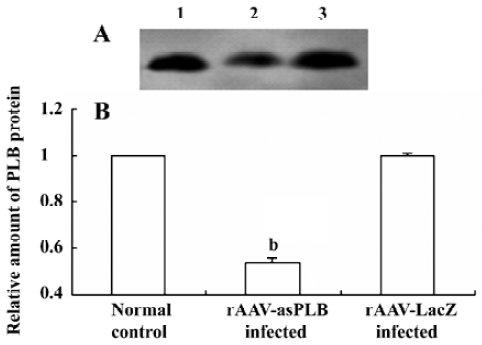
PLB protein expression in infected cardiomyocytes The level of PLB protein expression was decreased in rAAV-asPLB infected cardiomyocytes compared with rAAV-LacZ infected cardiomyocytes and normal control cardiomyocytes (P<0.05, Figure 4).
SERCA mRNA and protein expression and activity The level of SERCA mRNA and protein expression was not changed in rAAV-asPLB infected cardiomyocytes. However, the PLB/SERCA ratio was decreased because of a decrease of PLB expression. While the activity of SERCA was higher in rAAV-asPLB group than that in rAAV-LacZ group (2.04±0.09 vs 1.83±0.22 µmol·g-1 protein·min-1) and normal control (2.04±0.09 vs 1.84±0.03 µmol·g-1 protein·min-1) (P<0.05).
Effect of rAAV-asPLB on [Ca2+]i Resting state, fluorescent intensity of [Ca2+]i in rAAV-asPLB group (9.13±0.80) was lower than that in rAAV-LacZ group (13.82±0.80) and normal control (14.98±1.08) (P<0.05). Isoproterenol induced [Ca2+]i increase in cultured cardiomyocytes. The ΔF/F0 of [Ca2+]i in rAAV-LacZ group and normal control was 354% and 324%, respectively. However, the level of ΔF/F0 in rAAV-asPLB group was 459%.
Discussion
Heart failure represents an enormous clinical challenge in need of effective therapeutic approaches. The possibility of gene therapy for heart failure merits considerations at this time because of improvements in vector technology, cardiac gene delivery, and most importantly, our understanding of the molecular pathogenesis of heart failure. PLB is an endogenous muscle-specific inhibitor of SERCA2, cardiac-specific disruption of PLB in mice results in a marked increase in myocardial contractility[13]. In this study, PLB cDNA fragment was cloned and inserted inversely into pAAV-MCS, which constructed the plasmid vector of pAAV-asPLB.
Previous gene transfer approaches that used direct injection of plasmid DNA or replication-defective adenovirus vectors have been limited by low transduction frequencies and transient transgene expression due to immune response, respectively[14]. In this study, adeno-associated virus (AAV) was chosen as the gene transfer vector. In traditional AAV recombinant systems, the requirement for wild-type adenovirus co-infection, the contamination of adenovirus and the regeneration of wild-type AAV are major problems. However, the AAV Helper-Free system allows the production of infective recombinant AAV-2 virus without the use of a helper virus. Most of the adenovirus gene products required for the production of infective AAV particles are supplied on the plasmid pHelper that is co-transfected into 293 cells.
We constructed the rAAV vectors containing target gene (PLB) and reporter gene (lacZ) with AAV Helper-Free system. The rAAV vectors were identified and measured by PCR and in site β-galactosidase staining. Cardiomyocytes was infected with rAV-LacZ and stained with X-gal. The result shows that the rAAV is able to infect the cardiomyo-cytes effectively.
The antisense RNA can bind to its target RNA (sense RNA) thereby controlling expression of the target gene. In comparison to the ribozyme technique, antisense RNA technique has the advantages of simple designing, strong specialty, easy operating, and being economic and time saving[15]. In the present study, rat neonatal cardiomyocytes were cultured and infected by rAAV vectors. RT-PCR and Western blot results showed that the expression of PLB was decreased as compared with normal group and the rAAV-LacZ infected group. This indicated that the as PLB had partly blocked the transcription of PLB mRNA and inhibited the translation of PLB protein.
The PLB/SERCA ratio was decreased in rAAV-asPLB infected cardiomyocytes because the SERCA mRNA and protein expression had no change. While, the activity of SERCA was enhanced. Our results demonstrated that reducing PLB inhibition to SERCA enhanced the SERCA activity. Further study on [Ca2+]i showed the concentration of resting [Ca2+]i was decreased in rAAV-asPLB-infected cardiomyocytes. The [Ca2+]i was elevated after isoproterenol was induced. However, a higher [Ca2+]i was observed in rAAV-asPLB infected cardiomyocytes. These data suggested that asPLB enhanced the affinity of SERCA for Ca2+ and therefore improved diastolic/contractile function in rAAV-asPLB-infected cardiomyocytes.
Footnote
Project supported by the Scientific and Technological Agency of Zhejiang Province (N
References
- Bernecker OY, del Monte F, Hajjar RJ. Gene therapy for the treatment of heart failure-calcium signaling. Semin Thorac Cardiovasc Surg 2003;15:268-76.
- Zhai J, Schmidt AG, Hoit BD, Kimura Y, MacLennan DH, Kranias EG. Cardiac-specific overexpression of a superinhibitory pentameric phospholamban mutant enhances inhibition of cardiac function in vivo. J Biol Chem 2000;275:10538-44.
- Koss KL, Grupp IL, Kranias EG. The relative phospholamban and SERCA2 ratio: a critical determinant of myocardial contractility. Basic Res Cardiol 1997;92 Suppl 1:17-24.
- Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of β-agonist stimulation. Circ Res 1994;75:401-9.
- Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, Walsh RA, et al. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocytes mechanics in transgenic mice. J Clin Invest 1996;97:533-9.
- He H, Meyer M, Martin JL, McDonough PM, Ho P, Lou X, et al. Effects of mutant and antisense RNA of phospholamban on SR Ca2+-ATPase activity and cardiac myocyte contractility. Circulation 1999;100:974-80.
- Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation 2002;105:904-7.
- Clark KP, Voulgaropoulou F, Johnson P R. A stable cell line carrying adenovirus-inducible rep and cap genes allow for infectivity titration of adeno-associated virus vectors. Gene Ther 1996;3:1124-32.
- Zhang YB, Leng XS, Peng JR, He XJ. Construction, packaging, and titration of recombinant adeno-association virus vectors contaning antitumor genes. Natl Med J China 2002;82:564-7.
- Svensson EC, Marshall DJ, Woodard K, Lin H, Jing F, Chu L, et al. Efficient and stable transduction of cardiomyocytes after intramyo-cardiol injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation 1999;99:201-5.
- Larsen JS, Kjeldsen K. Quantification in crude homogenates of rat myocardial Na+, K+- and Ca2+-ATPase by K+ and Ca2+-dependent pNPPase age-dependent changes. Basic Res Cardiol 1995;90:323-31.
- Qiao GF, Zhou H, Li BY. Antagonistic effects of berbamine on [Ca2+]i mobilization by KCl, norepinephrine, and caffeine in newborn rat cardiomyocytes. Acta Pharmacol Sin 1999;20:292-6.
- Brittsan AG, Kranias EG. Phospholamban and cardiac contractile function. J Mol Cell Cardiol 2000;32:2131-9.
- Teramoto S, Ishii T, Matsuse T, Fukuchi Y. Recombinant adeno-associated virus vectors efficiently transduce foreign gene into bovine aortic endothelial cell: comparison with adenovirus vectors. Jpn J Pharmacol 2000;84:206-12.
- Wang L, Zhang GM, Feng ZH. Down-regulation of survivin expression reversed multidrug resistance in adriamycin-resistant HL-60/ADR cell line. Acta Pharmacol Sin 2003;24:1235-40.
