Validation of a simple automated movement detection system for formalin test in rats1
Introduction
The formalin test, first established by Dubuisson and Dennis[1], is a model of acute and tonic pain that is commonly used in studies of nociception in rodents and in the discovery of novel analgesics. The test has been characterized most extensively in rats, cats, and mice[1–3]. The injection of diluted formalin into the hindpaw of rats elicits a biphasic nociceptive behavioral response. This response is complex and includes such behavior as rearing, turning, jumping, crossings, flinching, shaking, elevating, clutching, and licking the affected paw and dysphoria. These responses have been most frequently monitored either by assessing a single, presumably representative, behavior (eg, the incidence of flinching, the duration of licking/biting)[4–7], or by using a weighted-scored measure[1] to evaluate the global response. However, these measurements, based on a subjective interpretation of the behaviors, are labor-intensive, time-consuming, tedious, and dependent on human judgement. The need to automate behavioral pain tests is obvious not only to facilitate their use but also to allow researchers from academic institutes and pharmaceutical companies to easily use more sophisticated and perhaps more predictive tests than the usual acute pain test. Therefore, different automated detection systems have been established for nociceptive behavior in the formalin test[8–10]. However, the computer-driven, dynamic-force automatic system[8] or the automated method of pain scoring[9–11] were difficult to purchase. Recently, a simple automated movement detection system has been described primarily by our laboratory to quantify the agitation responses elicited by subcutaneous (sc) formalin injection into the rats’ hindpaw[12]. The purpose of the present study was to further investigate the pharmacological sensitivity and validity of this system. The agitation responses elicited by different concentrations of formalin (50 µL) sc injected into the rat hindpaw were determined with this automated system, and then the effects of systemic morphine of different doses and local anesthetic lidocaine on the agtitation responses induced by the largest concentration of formalin (5%) were examined in the rat. As comparable control, manual measurement of the duration of licking/biting and the incidence of flinching of the injected paw were also simultaneously monitored in each test.
Materials and methods
SubjectsSixty adult male Sprague-Dawley rats weighing 260–280 g were provided by the Experimental Medical Animal Center of Shaanxi Province, China. Animals were housed in cages in groups of six with food and water available ad libitum on a 12 h light/dark schedule (lights on at 8:00 AM). The experiment was performed according to the Guidelines of the International Association for the Study of Pain[13] and approved by the Institutional Animal Care Committee of Xi’an Jiaotong University.
Formalin test Automated movement detection system The transformed part of the automated movement detection system consisted only of a spring balance (0–2.0 kg) and an electromagnetic transducer, a permanent magnet (1.5 cm×1.5 cm×1.0 cm) with an intensity of 0.5 T connected to the plane of the balance, which placed both the N and S poles of the magnet in a horizontal plane. One thousand five-hundred turns were wound on an E6 electric core and then installed in the magnetic field. Such an electromagnetic transducer was able to pick up and transform the mechanical movement of the spring balance plane into the induced current in the winding in the magnetic field, as shown in Figure 1. On the day of testing, each rat was moved to the laboratory and placed in a hexagonal polycarbonate chamber (L:16 cm; H: 8 cm) for about 15–20 min to acclimate it to the experimental environment. The criterion for adequate acclimation was that rats did not freeze or defecate in the test chamber[14] and rarely exhibited spontaneous activities. Then, the rat was talen from this chamber and formalin (50 µL) was administered sc into the hindpaw pad. The rat was immediately replaced in the chamber, which was placed on the spring balance with the electromagnetic transducer. Nociceptive behavior (agitation) elicited by the formalin injection such as flinching shaking, elevating, clutching, and favoring the affected paw, and dysphoria activities-induced movements of the balance, which was picked up and transformed into electrical signals via the electromagnetic transducer. The electrical signals were amplified, filtered (100 Hz), and displayed on an oscilloscope, and fed into a window discriminator to minimize the non-specific signals induced by spontaneous movement of the animal. A computer system that allowed quantitative recording of the number of agitation events and construction of time histograms over a 60-min observation period. To examine the different concentrations of formalin-induced agitation responses, rats were restrained lightly and received a single sc injection of saline (50 µL) or formalin (0.5, 1.5, 2.5, 5.0%, 50 µL, respectively) into the plantar surface of right hindpaw. Each rat was used only once and was killed by a lethal dose of pentobarbital at the end of the experiment.
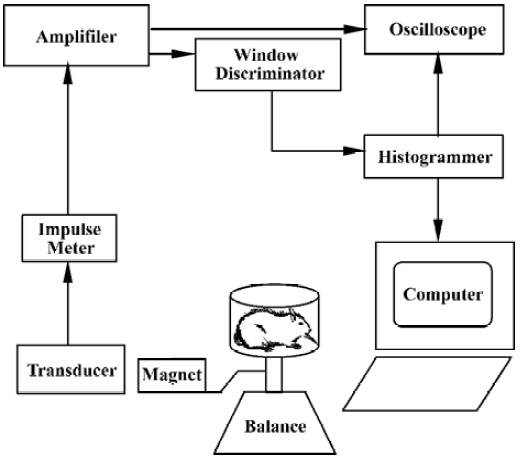
Manual detection method To evaluate the validity of the automated movement detection system for formalin test, the classical manual detection methods were also used synchronously. A mirror was positioned below the chamber at a 45º angle for unobstructed observation of the rats’ paws. The response to formalin injection was monitored by measuring the total duration(s) of licking/biting and the incidence (times) of flinching of the injected hindpaw over 5-min in the 60-min observation period, respectively, by two experimenters, as reported previously[4–6].
Pharmacological experimentTo determine the pharmacological validity of the automated movement detection system, the effects of sc injection of morphine hydrochloride of different doses (1, 2, and 5 mg/kg, dissolved in 0.9% saline) 10 min before formalin injection on the formalin-induced agitation responses were observed. In the subsequent experiments, the blocking effects of opioid receptor antagonist naloxone on morphine-induced inhibition of the agitation responses were further examined, ie, after administertion of the largest dose (5.0 mg/kg) morphine, which produced a maximal antinociceptive effect, naloxone hydrochloride (1.25 mg/kg, dissolved in 0.9% saline, Sigma, MO, USA) was administered sc just before formalin injection. The doses of morphine and naloxone were selected according to previous studies by using the weighted-scores method for behavioral measurement of nociception in the formalin test[8,15]. In another experimental group rats received an injection of lidocaine (2%, 20 mg/kg) into the ipsilateral ankle subskin of the rat 5 min prior to formalin (5%, 50 µL) injection to observe the effects of the local anesthetic on the formalin-induced agitation responses. The same volume of saline was injected before formalin injection in the control experiment. In all these experiments, after formalin injection, the nociceptive agitation responses were monitored for 60-min by using both the automated movement detection system and the manual detection method.
Data analysisData were expressed as the agitation event rate (Hz), the duration(s) of licking/biting and the incidence (times) of flinching of the injected paw per 5-min observation period. The correlation coefficient between the concentration or dose of drug and effect on nociceptive behavior was calculated with linear regression. Differences in drugs effects among groups were tested statistically by two-way repeated measures of analysis of variance (two-way RM ANOVA) with a post hoc multiple comparison (Bonferroni t-test) for analysis of the differences over the entire observation time or at each time point among different groups. P<0.05 was considered to indicate statistical significance.
Results
Nociceptive behavior induced by formalin injection to the hindpaw Injection of formalin into the rat hindpaws elicited a characteristic biphasic nociceptive agitation response when the response was monitored with the automatic system. The first phase (early phase) began immediately after formalin injection and lasted for approximately 5-6 min. After the first phase, there was one 6–10 min interphase, during which the rat was relative quiet in the chamber and rarely exhibited nociceptive agitation activities. The second phase (late phase) began approximately 10–15 min after formalin injection and lasted for approximately 40-50 min. In the second phase, the response peak appeared at about 35 min after formalin injection. The time histograms of the mean agitation events induced by formalin of different concentration (saline, 0.5, 1.5, 2.5, and 5.0%, 50 µL, n=6 for each treated group) was shown in Figure 2A–2E. The agitation event rate induced by 5.0% formalin during a 60-min observation period was significant larger than that of the saline, 0.5% or 1.5% formalin group (P<0.01, n=6) and that induced by 2.5% formalin was significantly larger than that of the saline group (P<0.05). Although there was no significant difference between the 5.0% and 2.5% formalin groups in overall effects, there were significant differences at three of 12 time points (P<0.05, Figure 2E). Linear regression analyses indicated that the nociceptive agitation response to formalin increased following the formalin concentration increase with a correlation coefficient (r) of 0.781 (P<0.01).
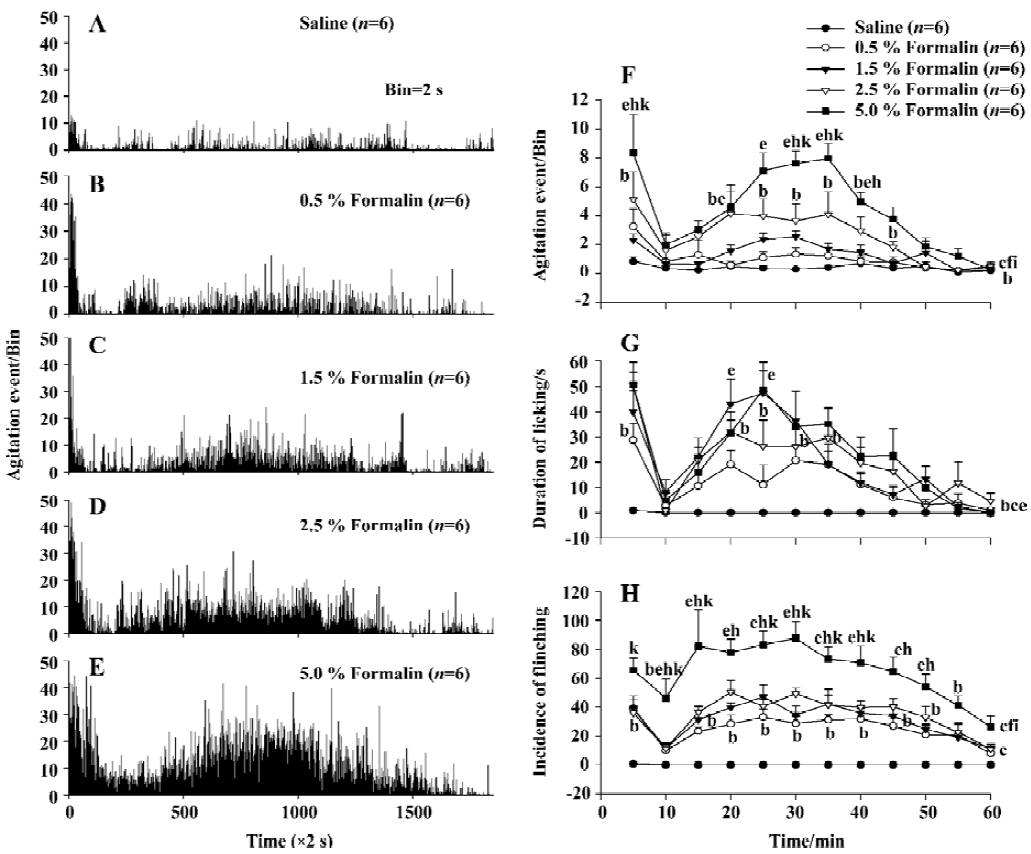
Similarly, the formalin-elicited biphasic response measured manually was comparable to that obtained with the automatic detection system (Figure 2G, 2H). These results were similar to previous reports using either automatic or manual methods[8,10,15].
Effect of morphine on formalin-elicited nociceptive behavior Injection of morphine (0, 1, 2, and 5 mg/kg) depressed the nociceptive agitation response to the largest dose (5%, 50 µL) of formalin injection in a dose-dependent manner with a correlation coefficient (r) of 0.876 as monitored by the automatic detection system (Figure 3A–3D). The inhibitory effect induced in both the 2 and 5 mg/kg morphine group during the 60-min observation period was significantly larger than that of the saline group (P<0.01, n=6). Although there was no significant difference between the 1.0 mg/kg morphine and saline groups (P>0.05, n=6) in the overall effect, the differences were significant at two of 12 time points (P<0.05). The inhibitory effect of the 1 mg/kg morphine group was significantly smaller than that of 2 mg/kg and 5 mg/kg morphine groups (P<0.05) at the 35 min time point after formalin injection (Figure 3E).
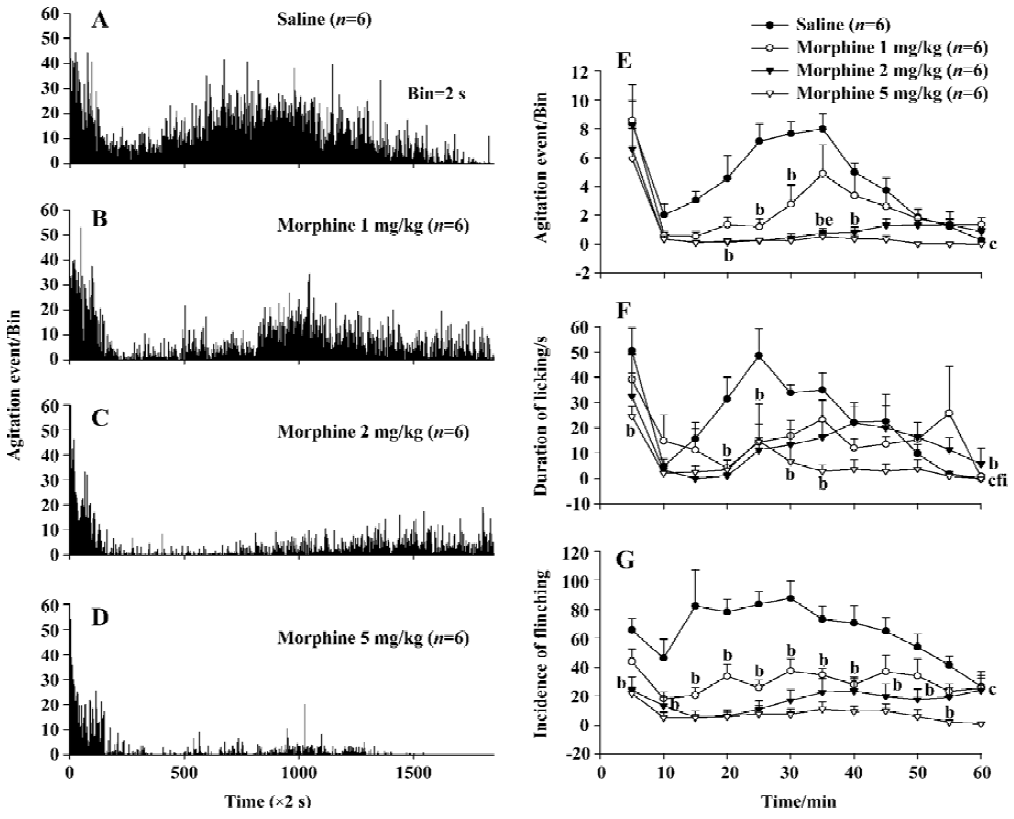
The inhibitory effect of morphine to the formalin-evoked nociceptive behavior was also measured manually. The decreases in the licking/biting duration induced in the 2 and 5 mg/kg morphine groups were significantly different from that of the saline group (P<0.05, P<0.01, n=6) during the 60-min observation period. At two time points the effect in 1 mg/kg morphine group was different from saline group (P<0.05). The inhibitory effect of 5 mg/kg morphine was significantly different from those of the 1 and 2 mg/kg morphine in overall effects (P<0.01, n=6). Similarly, the decreases in the flinching incidence in the 1, 2, or 5 mg/kg morphine group were all different from the saline group (P<0.01, n=6). However, no-significant differences were found among the three morphine groups either in the entire effects or at any time point, as shown in Figure 3F, 3G.
Effect of naloxone on morphine-induced inhibition of nociceptive behavior in the formalin test Injection of the opioid receptor antagonist naloxone 1.25 mg/kg after morphine (5.0 mg/kg) application completely antagonized the morphine-evoked inhibition of agitation responses induced by formalin injection (Figure 4A–4C). There was statistically significant difference (P<0.01, n=6) between the effects induced by naloxone plus morphine and those by morphine alone, but no significant difference (P>0.05) was detected between effects in the naloxone plus morphine group and the saline group (Figure 4E).
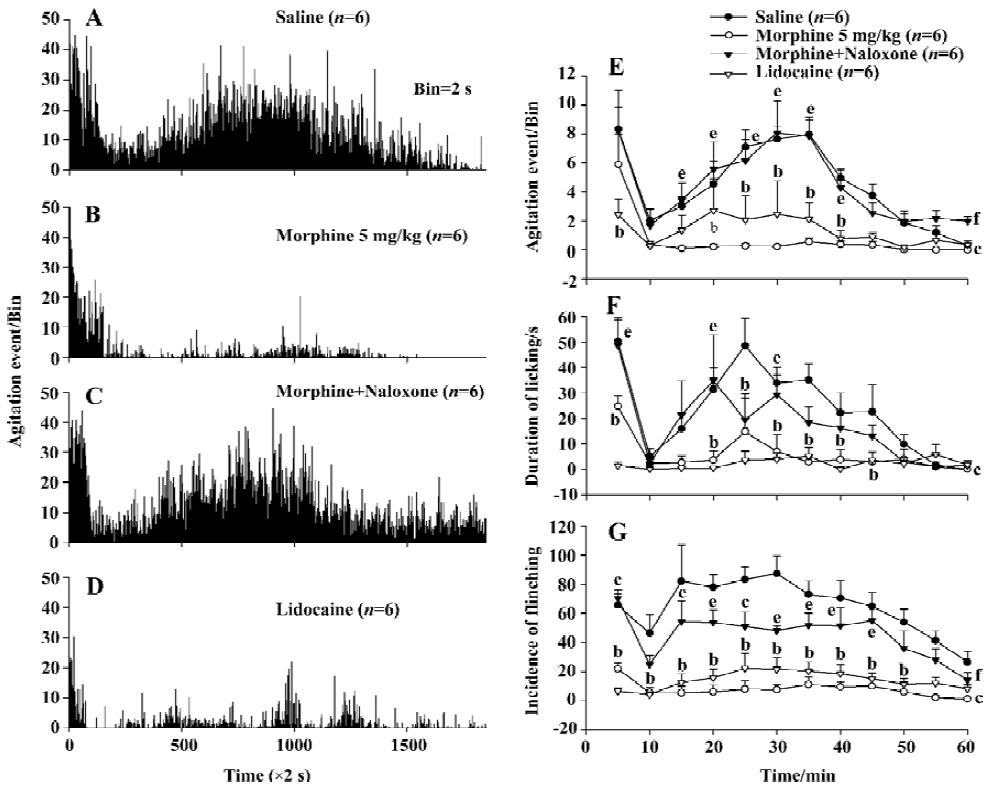
When manual detection methods were used, naloxone significantly blocked the decrease in the licking/biting duration (P<0.01) and flinching incidence (P<0.01) elicited by morphine (Figure 4F, 4G).
Effect of lidocaine on the nociceptive behavior induced by formalin Injection of lidocaine 20 mg/kg into the ipsilateral ankle subskin completely blocked the agitation response to formalin injection as measured by the automatic system. There was a statistically significant difference (P<0.01) between the effects in saline group and the lidocaine group (Figure 4A, 4D).
When manual detection methods were used, there were also significant differences in the duration of licking/biting (P<0.01) and flinching incidence (P<0.01) between the saline and the lidocaine groups (Figure 4F, 4G).
Discussion
The present study found that a typical biphasic nociceptive behavior (agitation) induced by formalin injection into the rat hindpaw could be detected by the automated movement detection system developed by our laboratory[12], and that this response was proportional to those obtained from manual scoring method both in the time course and the magnitude of change. It is identical to previously reports about the formalin test in rats[1,9,10], either using a weighted-scores measurement or a single nociceptive behavior measurement such as licking/biting or flinching the injected paw. This indicates that the simple automated movement detection system for quantifying the nociceptive behavior elicited in the formalin test is a validated measure and produces results that are comparable to those found using a computer-driven, dynamic-force automatic detection system[7] or a new automated method of pain scoring[9,11].
The first phase of response elicited in the formalin test was believed to be a result of direct activation of peripheral nociceptors, whereas the second was mediated by a combination of low ongoing activity in primary afferents and increased sensitivity of spinal cord neurons (inflammatory hyperalgesia)[16,17]. Therefore, the intensity of the agitation response to formalin recorded by using the automatic system should be of a stimulation-response relationship. The results of this study demonstrated that the nociceptive agitation response increased linearly following the increase of stimulation intensity with a greater correlation coefficient which is similar to the results that obtained from manual detection methods. These results were also consistent with those obtained by using a weighted-scores method of behavioral pain rating[4] and using a computer-driven, dynamic-force automatic detection system[7]. Therefore, the results reinforced the validity of the new automatic detection system for monitoring the nociceptive response to formalin injection.
The validity of the automatic system was further confirmed by the results obtained from systemic morphine-evoked analgesia. The nociceptive agitation response to formalin as monitored by the automatic detection system was inhibited by sc injection of morphine in a dose-dependent manner which was similar to that described in other studies[8,9,15]. The antinociceptive effect of the largest dose (5.0 mg/kg) of morphine could be effectively antagonized by opioid receptor antagonist naloxone. These results were also comparable to those obtained using manual methods suggesting that the automatic system has a good sensitivity to analgesics and therefore provides a good measurement to evaluate the effect of analgesics.
In the present study, the data obtained with both the automatic detection system and manual methods indicated that the nociceptive response could be completely blocked by pre-administration of the local anesthetic lidocaine. The results are identical to those reported previously[18,19]. It has been demonstrated that intraperitoneal injection of lidocaine (10–30 mg/kg) induced significant antinociceptive effect in both phases of the formalin test using the paw-licking measurement[18]. A pre-injection of lidocaine (sc) into the ankle before formalin could markedly decrease the formalin-evoked Fos-immunoreactivity in the dorsal horn neurons[19]. These studies showed that the automated movement detection system provided a good pain measurement and a pharmacological validation for the system. The results correlate well with the licking duration and incidence of flinching measures for invention and detection of new analgesic drugs.
In summary, the data from the present study show the validity of an automatic detection system with following advantages: (1) it is an objective measure; (2) global analysis of pain-induced behaviors superior to the recording of a single parameter such as licking duration or flinching incidence; (3) the system is less time consuming spent by the experimenter; (4) that the new automatic detection system could be used in pharmacological studies in inflammatory pain; and (5) the transducer equipment in the automatic system is very simple and cheap. In a general electrophysiological laboratory, this system can be easily made and used in studies of nociception and antinociception. Although the automatic system cannot clearly distinguish the agitation response and the spontaneous activities, the latter can be reduced to smallest if the animal adequately habituated to the experimental arena and were kept quiet in the chamber before formalin administration.
Footnote
Project supported by the National Natural Science Foundation of China (N
References
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brainstem stimulation in rats and cats. Pain 1977;4:161-74.
- Murrary CW, Porreca F, Cowan A. Methodological refinements to the mouse paw formalin test: an animal model of tonic pain. J Pharmacol Methods 1988;20:175-86.
- Yang Q, Lu JT, Zhou AW, Wang B, He GW, Chen MZ. Antinociceptive effect of astragalosides and its mechanism of action. Acta Pharmacol Sin 2001;22:809-12.
- Manning BH. A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala. J Neurosci 1998;18:9453-70.
- Noble F, Blommaert A, Fournie-Zaluski MC, Roques BP. A selective CCKB receptor antagonist potentiates µ-, but not δ-opioid receptor-mediated antinociception in the formalin test. Eur J Pharmacol 1995;273:145-51.
- Przewlocka B, Mika J, Labuz D, Toth G, Przewlocki R. Spinal analgesic action of endomorphines in acute, inflammatory and neuropathic pain in rats. Eur J Pharmacol 1999;367:189-96.
- Wheeler AH, Cowan A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology 1991;104:35-44.
- Jett MF, Michelson S. The formalin test in rats: validation of an automated system. Pain 1996;64:19-25.
- Jourdan D, Ardid D, Bardin L, Bardin M, Neuzere D, Lanphouthacoul L, et al. A new automated method of pain scoring in the formalin test in rats. Pain 1997;71:265-70.
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, et al. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol 2001;90:2386-402.
- Jourdan D, Alloui A, Eschalier A. Pharmacological validation of an automated method of pain scoring in the formalin test in rats. J Pharmacol Toxicol Methods 1999;42:163-70.
- Li Y, Yuan B, Tang JS, Xiang RK. A simple automated detecting system for formalin test in rats. Chin J Neurosci 2001;17:145-8.
- Zimmermann M. Ethical guideline for investigations of experimental pain in conscious animals. Pain 1983;16:109-10.
- Abbott FV, Franklin KBJ, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain 1995;60:91-102.
- Coderre TJ, Fundytus ME, McKenna JE, Dalal S, Melzack R. The formalin test: a validation of the weighted-scores method of behavi-oural pain rating. Pain 1993;54:43-50.
- Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurons in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain 1987;30:349-60.
- Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 1992;263:136-46.
- Bittencourt AL, Takahashi RN. Mazindol and lidocaine are antinociceptive in the mouse formalin model: involvement of dopamine receptor. Eur J Pharmacol 1997;330:109-13.
- Tokunaga A, Doi M, Senba E. Effects of local anaesthesia on formalin-induced Fos expression in the rat dorsal horn. Neuroreport 1995;26:2301-4.
