Inhibitory effect of picroside II on hepatocyte apoptosis1
Introduction
Picrorhiza scrophulariiflora belongs to the plant family, scrophularia. Dried rhizome from Picrorhiza scrophularii-flora is used for medicine benefits. Many published studies have found that it can protect hepatocytes from many kinds of injury, but the mechanism has not been clearly elucidated. Picroside II is one of the most effective components extracted from Picrorhiza scrophulariiflora. To investigate the mechanism of picroside II’s protective effects on hepatocytes, we have established hepatocyte apoptosis models in vivo and in vitro. Transmission electron microscopy, agarose gel electrophoresis of DNA and flow cytometry were used to evaluate apoptosis of hepatocytes. Semi-quantitative RT-PCR together with image manipulation were used to analyze bcl-2 and bax mRNA which are highly related to apoptosis; immunohistochemistry was used to observe the protein expression of Bcl-2 and Bax. Together with several other oxidation indexes, we tried to demonstrate the mechanisms of picroside II’s effect on liver protection in apoptosis.
Materials and methods
Animals Seventy-two mice of Kunming strain (20±2 g) and 2 SD rats (70±10 g) were supplied by the Animal Center of Academy of Military Medical Sciences (Beijing China), with the number of males and females being equivalent.
Materials Picroside II was supplied by the Bescholor Research Center of Peking University (Beijing China). Dicarboxylate pilules (DDB) were obtained from Beijing Union Pharmaceutical Factory (Beijing China). D-galactosamine (D-GalN), lipopolysaccharide (LPS), actinomycin-D (Act-D), and tumor necrosis factor-α (TNF-α) were purchased from Sigma (St Louis, Mo, USA). RNase A, diethylpyrocarbonate (DEPC), fetal bovine serum (FBS), and Trizol reagent were purchased from Gibco/BRL (Cali-fornia, USA). The Bcl-2 monoclonal and Bax polyclonal antibodies were purchased from Santa Cruz Biotechnology (California,USA). Reverse Transcriptional kit was obtained from Promega Chemical Co (Wisconscin,medison,USA). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) detection kits, malondialdehy (MDA, Mn-Super oxide dismutase (Mn-SOD), and streptavidin-peroxidase (SP) were all domestic products.
Models establishing and treatment
In vivo Before experiments were started, 72 mice were divided into 6 groups randomly. Five groups were treated with D-GalN (700 mg/kg, ip) and LPS (1 µg/kg, ip) to establish hapatocyte apoptosis model in vivo, among which the 4 groups were infused (ig) with picroside II 5 mg/kg, 10 mg/kg, 20 mg/kg, or vehicle (0.9% NaCl) 10 min before and 4 h after the D-GalN and LPS administration, respectively. One group received biphenyl dimethyl dicarboxylate pilules (DDB) (200 mg/ kg) with the same manipulation acts as a positive control. The remained group was the negative control. Five hours after the model was established, the blood of 6 mice from each group was sampled though eye socket veins. ALT and AST in serum were then monitored (by continuously using the monitoring method, recommended by National Clinical Chemistry League). Cut sections of liver tissue (left part) of the remaining 6 mice of each group were prepared for pathology evaluation and immunohistochemistry detection of Bcl-2/Bax proteins. The liver suspending liquid (20%) was made from the rest of the liver and stored at under -20 ºC, in order to detect SOD in liver mitochondria (by the Marland method), protein level (by the Lowry method) and the content of MDA of liver tissue (by TBA colorimetry). Another 1 mL of liver suspending liquid was centrifuged at 12 000×g for 20 min. 400 µL upper liquid, 1 mL ethanol and 50 µL (5 mol/L) was mixed together and stored at under -20 ºC for DNA fragmentation analysis.
In vitro Primary culture of hepatocytes from rats were isolated as previously described[1]. Rats were decapitated under anesthesia. In situ perfusion was then performed with calcium and magnesium-free Hanks’ salt solution followed by a medium containing collagenase through the hepatic portal vein of rats, the suspending liquid containing rat liver cells were collected. The suspending liquid was then centrifuged at 120×g at 4 ºC for 5 min. The cell pellets were washed twice with cold Hanks’ solution and the cell concentration was modulated to 1×109 /L. The cells were seeded in 24-well culture plates in the growth medium consisting of DMEM with 20% FBS, at 37 ºC in a water-saturated atmosphere of 5% CO2. After 24 h, the culture medium was abandoned, and cells were exposed to a series of concentrations of picroside II (0.005, 0.01, and 0.02 mmol/L). After 1 h, Act-D 50 μg/L was added, and 30 min later TNF-α 3000 kU/L was added consecutively to the well for another 24 h. A negative control group was set which was exposed to the normal culture medium. An assessment of hepatocyte apoptosis and apoptosis-related genes bcl-2 and bax mRNA extraction was then performed.
The Marland method was used to detect the content of SOD in liver mitochondria. MDA content of liver tissue was detected by TBA colorimetry and the protein level of liver was analyzed using Lowry method . ALT and AST in serum were detected by the continuous monitoring method (recommended by International Clinical Chemistry League).
Immunohistochemistry analysis Liver tissues were fixed in 4% formaldehyde for 24 h and embedded in paraffin routinely, followed by 5 µm slicing up. The streptavidin-peroxidase (SP) method was assumed. The bcl-2 monoclonal antibody (1:50) or Bax polyclonal antibody (1:50) as primary antibodies was applied and incubated overnight at 4 ºC or for 60 min at room temperature. The slices were observed and photographed through light microscope and the percentage of the positive staining area of bcl-2 or bax were semiquantitatively analyzed by using CMIAS-008 Image Analyser (Beijing, China).
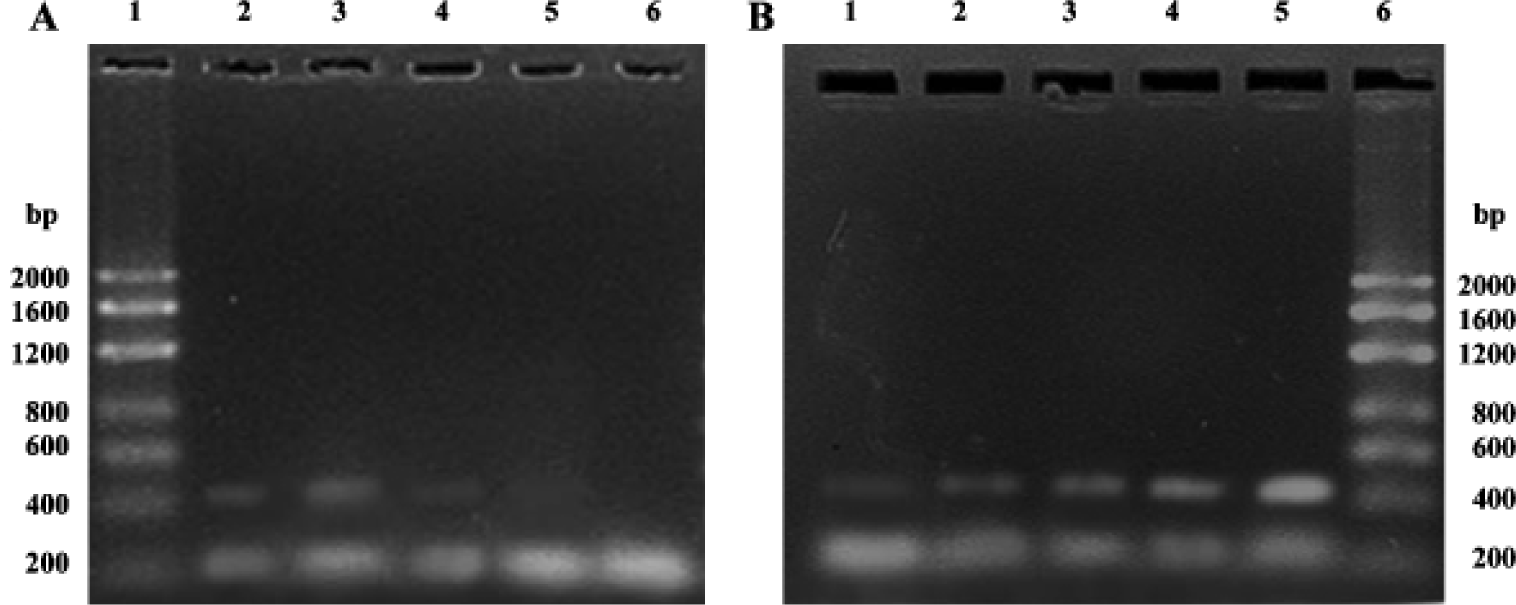
Reverse transcriptase polymerase chain reaction assay Total cytoplasmic RNA of each group was isolated from 1.5×107 cultured cells by the TRIZOL method (according to GIBCO specification). The concentration of RNA was determinated by absorbance at 260 nm, and its integrity was confirmed by means of electrophoresis on 1% agarose gels, and then stained with 0.1 mg/L ethidium bromide (EB). A total of 1 µg of RNA was converted to complementary DNA (cDNA) using 15 U reverse transcriptase in 20 µL buffer, which contained 1 mmol/L deoxy-NTP, 1 U RNase inhibitor and 0.5 µg oligo (deoxythymidine)15 primer. An aliquot (5%) of cDNA was amplified using the reverse (5’-AGA GGG GCT ACG AGT GGG AT-3’) and forward primer (5’-CTC AGT CAT CCA CAG GGC GA-3’) to yield 450 bp polymerase chain reaction (PCR) product for bcl-2, the reverse (5’-GGT TTC ATC CAG GAT CGA GAC GG-3’) and forward primer (5’-ACA AAG ATG GTC ACG GTC TGC C-3’) to yield a 429 bp PCR product for bax. In order to verify the equal amounts of cDNA were presented in the PCR reaction, β-actin was separately co-amplified with the bcl-2 and bax cDNA, with the reverse (5’-TAA AGA CCT CTA TGC CAA CAC AGT-3’) and forward primer (5’-CAC GAT GGA GGG GCC GGA CTC ATC-3’) to yield a 240 bp PCR product. The PCR conditions were as follows: pre-denaturation at 94 ºC for 5 min, denaturation at 94 ºC for 30 s, annealing at 60 ºC (bcl-2 and β-actin) or 64 ºC (bax) for 40 s, and polymerisation at 72 ºC for 40 s with Taq DNA polymerase. After 28 cycles, 20 µL bcl-2 or bax PCR product together with the β-actin PCR product of the same template were separated by electrophoresis and revealed by EB staining. Then each band of the electrophoresis gel was semiquantitatively analyzed using Imagemaster and Software Image Analyser (Beijing,China). The bcl-2 and bax mRNA levels were expressed as the ratio of signal intensity for the target genes in relation to that for the coamplified β-actin.
Flow cytometry analysis Cultured hepatocytes 1×109/L of each group were harvested, washed with PBS at 4 ºC, and exposed to pre-chilled ethanol at 4 ºC for 30 min. They were then washed again with PBS, exposed to PI 50 mg/L, 0.1% TritonX-100, 0.01 mmol/L EDTA (Na)2 and RNase 50 mg/L at normal temperature in darkness for 12–24 h. Specimen were then presented to the FACS-420 Flow Cytometry Analyser (New York, Becton Dickinson and Company, American) to evaluate apoptosis levels.
DNA fragmentation analysis The cultured hepatocytes of each group were lysed in Tris-HCl 20 mmol/L (pH 8.0), EDTA 10 mmol/L, and 0.5% lauryl sarcosyl containing 200 mg/L of proteinase K. DNA was extracted by supercen-trifugation, precipitation with ethanol and was treated with RNase. The DNA was then loaded onto 1%–2% agarose gel containing ethidium bromide (EB), which was observed and photographed under UV transillumination.
Morphological evaluation light microscope assay Serial slices of liver tissues were prepared from mice in each group and stained with hematoxyline-eosin (HE) and then observed under light microscope at 200× or 400×magnification.
Transmission electron microscopy assay Liver tissue specimen were fixed and postfixed with glutaraldehyde and osmium troxide respectively, dehydrated by acetone, imbedded in eposy resin. Ultrathin sections were collected and counterstained with lead citrate and viewed with a JEM100CXtransmission electron microscope (Tokyo, Japan).
Statistical analysis All results were expressed as mean±SD. Data were assessed by using t-test, and P<0.05 was considered stastically significant.
Results
Effects of picroside II on ALT and AST in serum of mice suffered from acute liver injury induced by D-GalN and LPS ALT and AST values were markedly higher in the model group than those in negative control (P<0.01). Picroside II decreased ALT and AST values in a dose-dependent manner (P<0.05 or P<0.01 vs model control). ALT and AST values of the positive control group treated with DDB 200 mg/kg also decreased, especially ALT. The results revealed that picroside II can protect liver tissue from D-GalN and LPS-induced acute injury, and it was more powerful than DDB (Table 1).
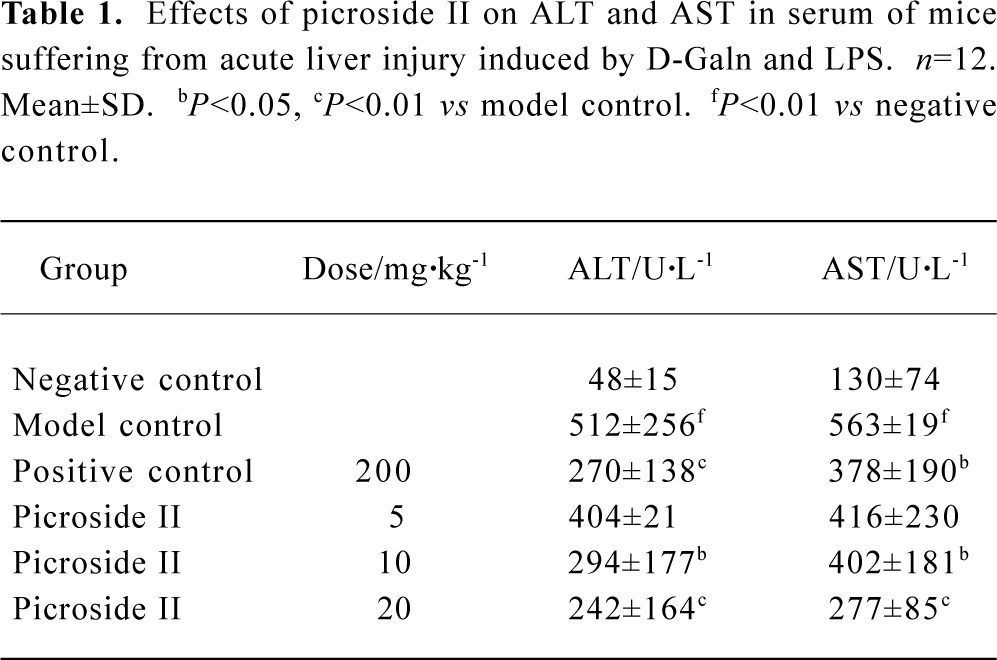
Full table
Effects of picroside II on MDA and SOD content of mice suffered from acute liver injury induced by D-GalN and LPS The SOD value of model group decreased, but the MDA value increased compared with negative group (P<0.01). The content of MDA of all groups exposed to picroside II markedly decreased, whereas SOD increased compared with the model group. This effect suggested a dose-dependent manner (P<0.05 or P<0.01) (Table 2).
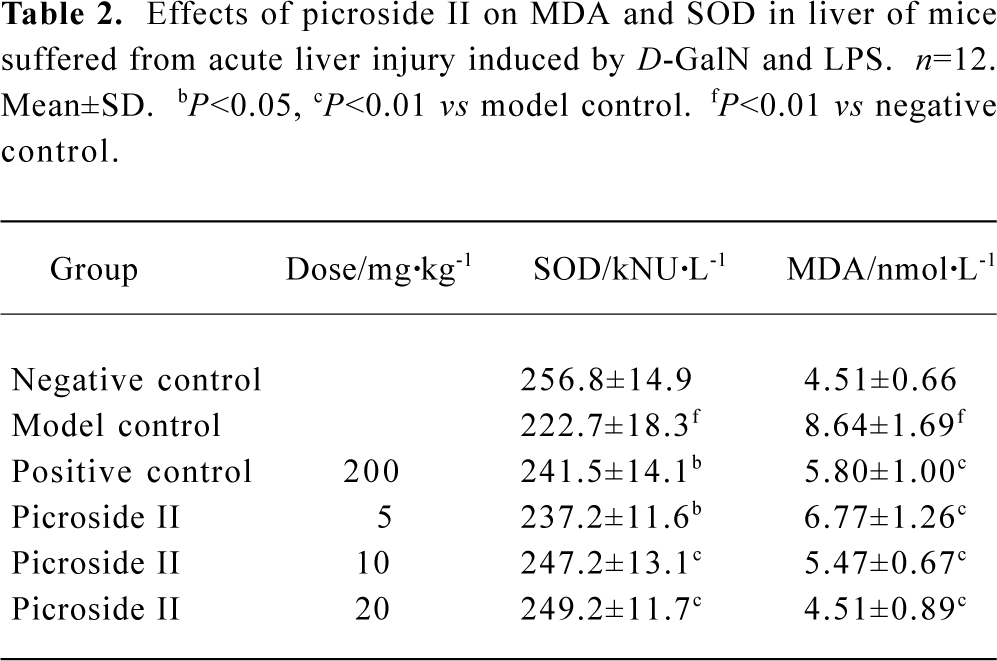
Full table
Immunohistochemistry analysis of Bcl-2 and Bax A few pale yellow granula (Bcl-2 protein) were detected in hepatocytes cytoplasm of the negative group. There were more granula in the model group and the most were found in the groups treated with picroside II (Figure 1A). Little buff granula (Bax protein) was detected in normal hepatocytes. The amount of the granula was more in the model group, and existed dispersedly or in focus with the color more heavily than others. Bax protein expression in groups treated with picroside II decreased (Figure 1B). The difference between groups treated with picroside II and the group of negative control was evidently significant (P<0.05 or P<0.01). The difference between picroside II groups and model control group was also significant (P<0.05 or P<0.01, Table 3).
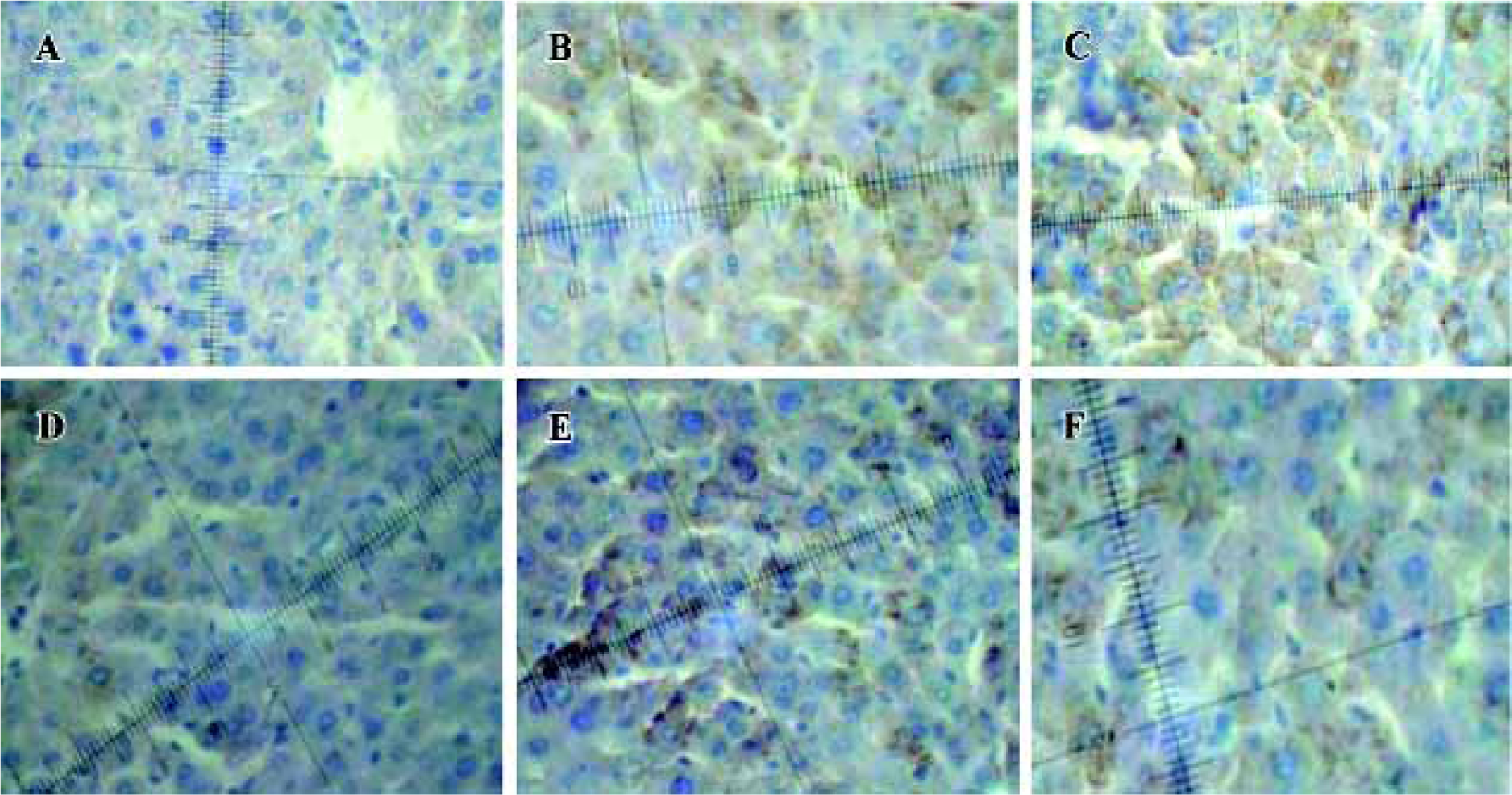
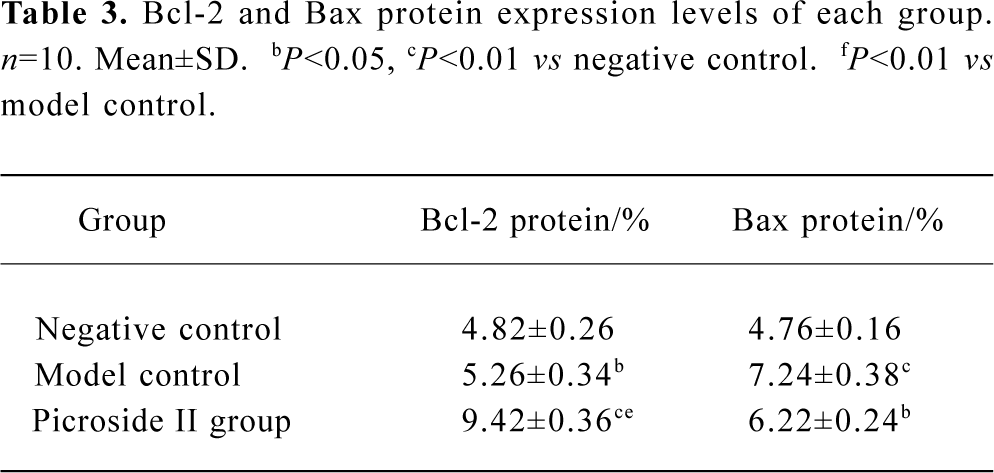
Full table
These results suggested that picroside II could effectively up-regulate the expression of apoptosis-related gene bcl-2 and inhibit bax expression. The increased bcl-2/bax ratio, thereby counteracted the hepatocyte apoptosis.
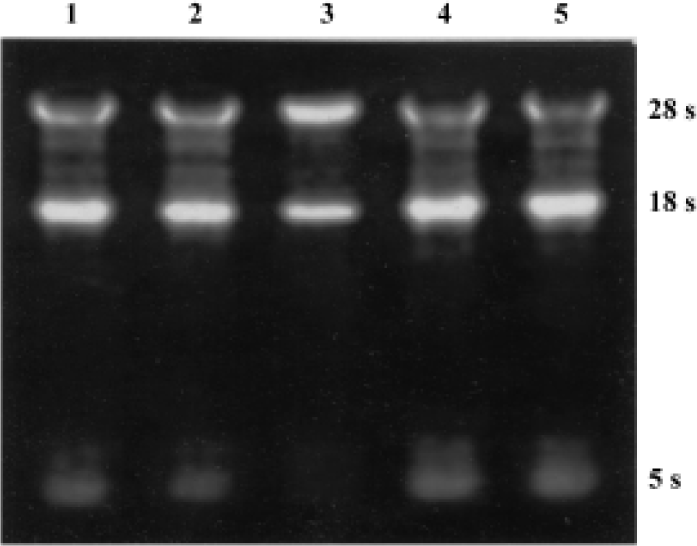
Semiquantitative RT-PCR analysis of apoptosis-related genes bax and bcl-2 mRNA The total RNA extracted from each group were loaded onto 1% agarose gel; 28 s, 18 s, and 5 s bands could be clearly observed, which confirmed the integrity of the total RNA (Figure 2). In the present study, 20 µL bcl-2 or bax PCR product together with β-actin PCR product of the same template were separated by electrophoresis and revealed by EB staining. PCR products of bcl-2, bax, and β-actin were 450 bp, 429 bp, 240 bp, respectively (Figure 3). The bcl-2 mRNA was the most in the group treated with picroside II 20 g/L, and the least in the negative group; bax mRNA was the most in model group, followed by the negative group, and the least in the groups treated with picroside II.
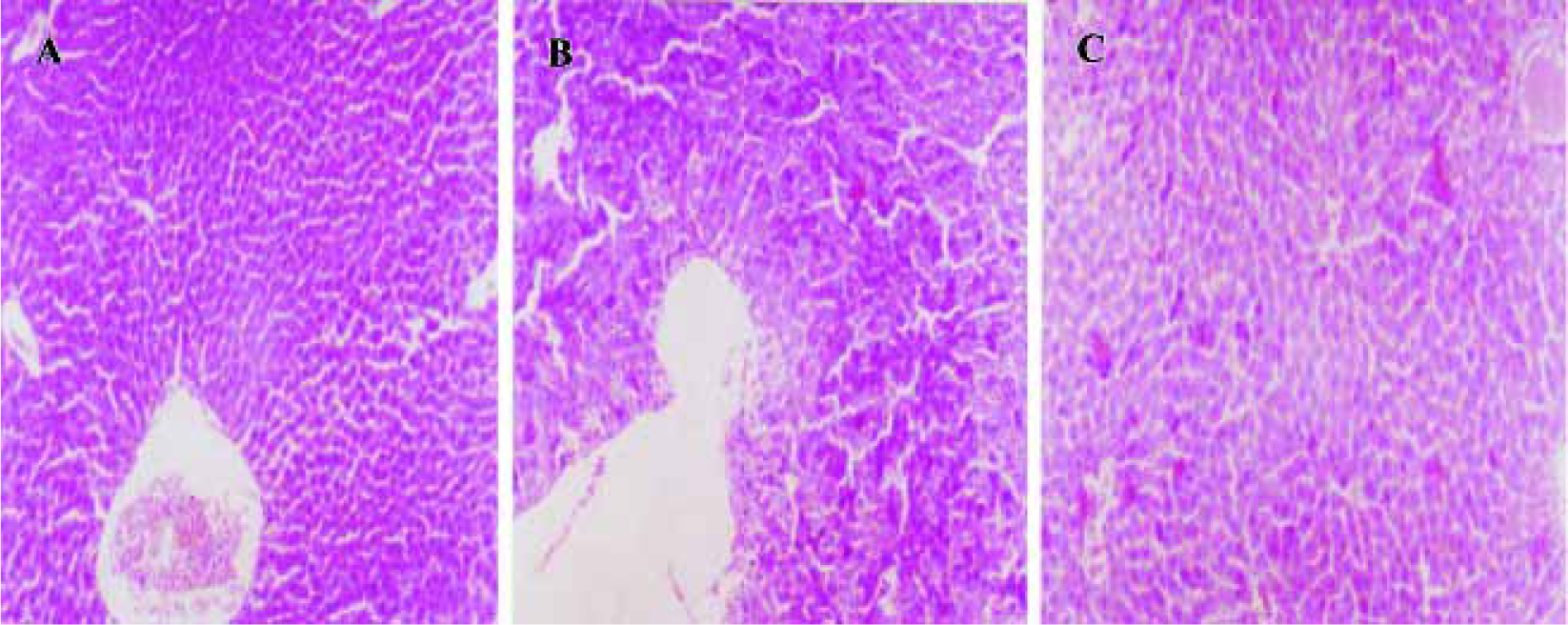
Morphological evaluation by light microscope assay Hepatocytes exhibited acidophilic trait induced by D-GalN and LPS, and there were acidophilic bodies in cytoplasm. There was no obvious pathological change observed in groups treated with picroside II 10 and 20 g/L (Figure 4).
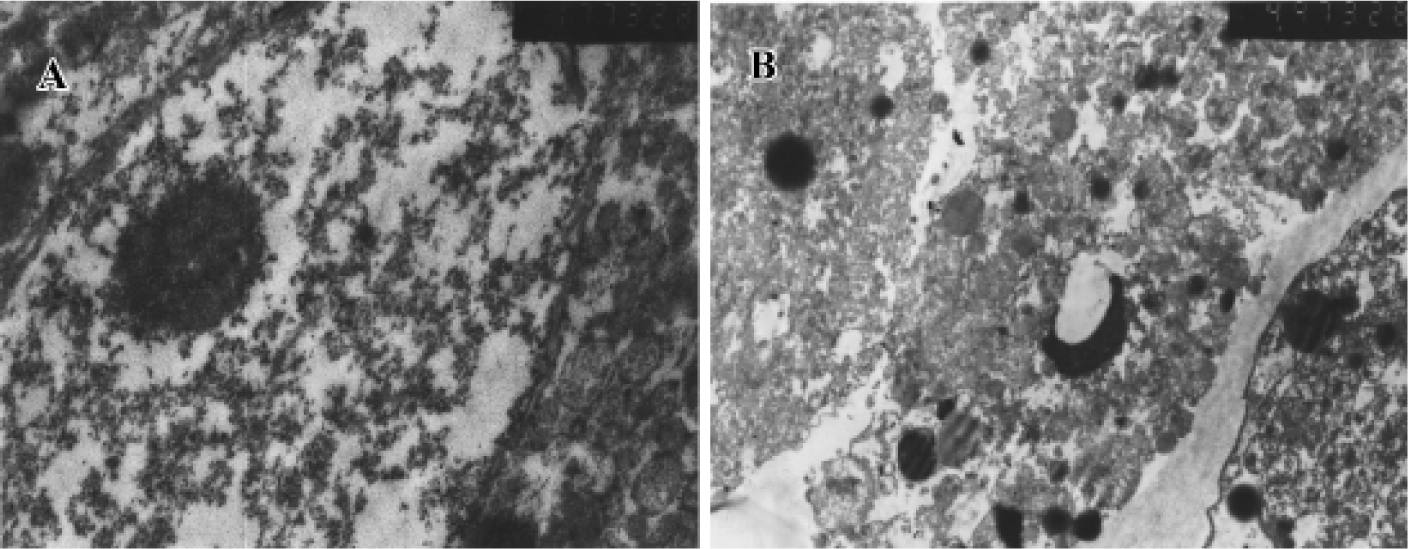
Transmission electron microscopy assay Normal hepatocyte was capsule intact, cytoplasm well-diffused, and karyotheca distinct and bears large nuclei. Apoptotic cells in the initial stages had shrunken and compacted with condensed or marginal chromatin. Chromatin spalls to pieces or semilunar body gradually and cytoplasm becomes condensation. In addition, blebbing of the plasmalemma, forming apoptosis body, a hall marker of apoptosis was then be observed (Figure 5).
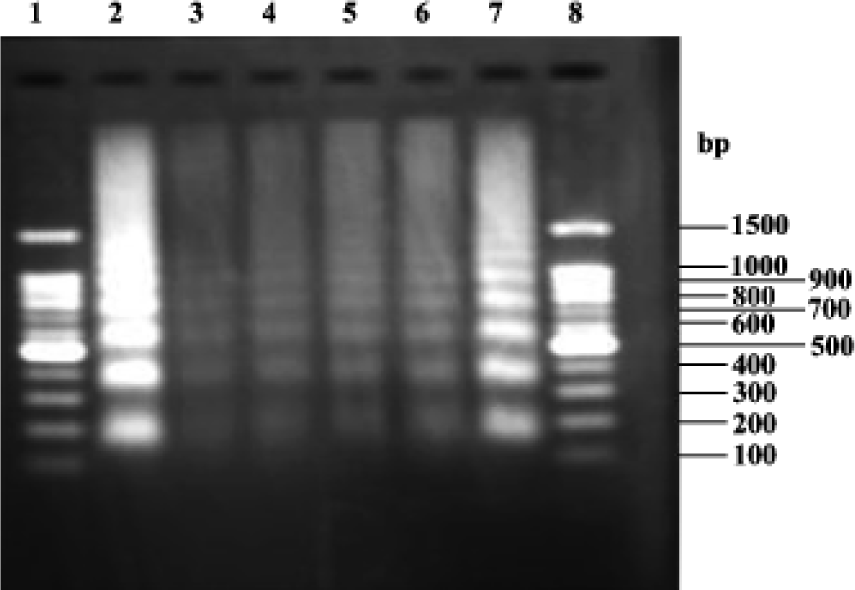
DNA fragmentation analysis Figure 6 shows that the model groups (lanes 2 and 7) exhibited the characteristic DNA ladder. In the groups treated with picroside II 5, 10, 20 g/L (lane 6, 5 and 4, respectively), the DNA ladder became less characteristic. Groups treated with picroside II 20 g/L (lane 4) were similar to negative group (lane 3).
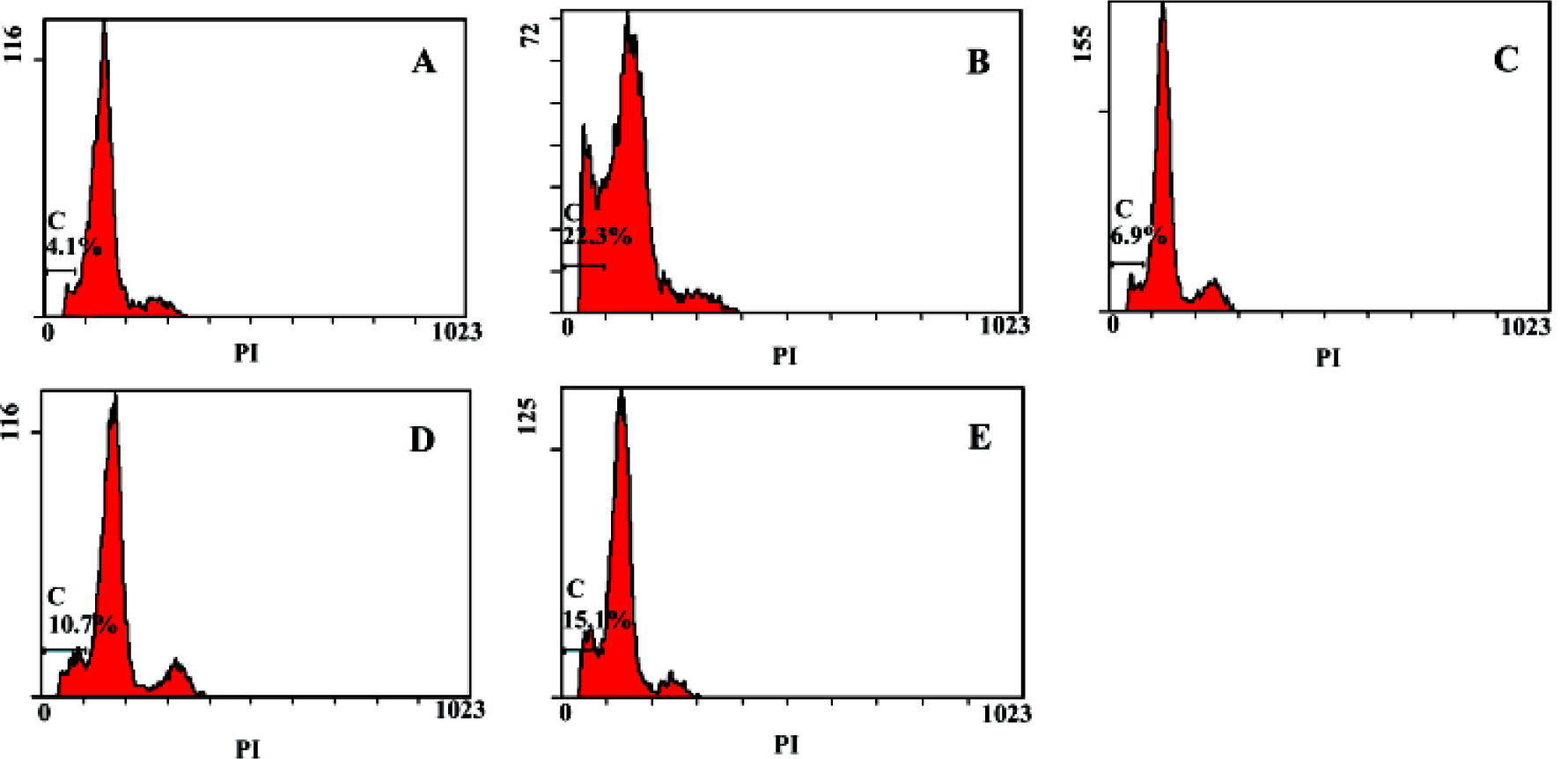
Flow cytometry analysis of apoptosis Apoptosis rate of primary hepatocytes of the negative group was 4.1%, the model group was 22.3%, groups treated with picroside II 20, 10, 5 g/L were 6.9%, 10.7% and 15.1%, respectively, which suggested that picroside II could protect hepatocyte against apoptosis, and it acted in a dose-dependent manner (Figure 7).
Discussion
The pathogenesis mechanisms of viral hepatitis is closely related to hepatocyte necrosis as well as apoptosis closely. Invasion of hepatitis virus to liver or hepatocytes canceration will result in hepatocyte apoptosis[9–11]. In inflammtory hepatitis, acidophilic body (shrunken necrosis, apoptosis corpuscula) and piece-meal necrosis were the characteristic morphological manifestation of apoptotic hepatocytes. Apoptosis of hepatocytes infected by virus can be a protective mechanism of the host to protect against virus replication and distribution from the infected cells[12]. Apoptotic cell death contributes to the removal of infected or cancerized cells, but over-apoptosis results in fulminate hepatitis[13,14]. Commonly, drugs are used to block hepatocyte apoptosis in order to cure hepatitis gravis, and to induce hepatocyte apoptosis for removing infected and cancerized cells[15–20].
One important molecular mechanism for cell apoptosis might be the modulation of the expression of bcl-2 gene family, which plays a critical role for the common pathway of apoptosis. Previous studies showed that proteins of Bcl-2 family could inhibit apoptosis of chronic infective hepatocytes, marrow stem cells, and nerve cells. The biological function of Bcl-2 is to increase the power to resist many apoptotic factors, counteract the effect of apoptosis, and extend lifespan. Bax, another important apoptosis-modulating gene product, is highly related to Bcl-2. Bax inactivates Bcl-2 by binding it to form a heterogeneous dimer. The mRNA and protein ratio of Bcl-2 to Bax is a pivotal factor in determine whether or not apoptosis can happen to cells exposed to many injuries [21]. Studidies have revealed that TNF-α could induce hepatocyte apoptosis, thus to participate in the onset of liver diseases. However, TNF-α itself dose not act in vivo and in vitro unless it is used together with Act-D and D-GalN[22]. Act-D is an inhibitor of RNA transcription, and could sensitize cells to the toxicity of TNF-α[23]. Therefore, the combination of Act-D and TNF-α is frequently used to establish models of hepatocyte apoptosis in vivo as well as in vitro[24] . The present study assumed that Act-D together with TNF-α to the establish experimental apoptosis model, agarose gel electrophoresis of DNA, and flow cytometry were used to evaluate hepatocyte apoptosis; light and transmission electron microscopy were used to observe morphological changes of cells and tissues; and quantitatively analysed the hepatocytes apoptosis. These results indicated that Picroside II evidently inhibited the morphological changes of hepatocytes, DNA fragmenta-tion, and the increase of sub-G1 spike (represents proportion of apoptosis). Immunohistochemistry and semi-quantitative RT-PCR analysis of the Bcl-2 and Bax expression and mRNA of bcl-2 and bax genes suggested that both bcl-2 and bax increased after hepatocyte injury, especially bax. The bcl-2 level and the ratio of bcl-2/bax increased markedly in groups treated with picroside II and apoptosis changes were also inhibited.
MDA is a lipid peroxide, whose content usually reflects the level of lipid peroxidation and indirectly reflects the extent of injury in vivo. SOD is a pivotal factor influencing the balance of oxidation and antioxidation, and its activity could indirectly reflect the capability of removing oxygen free radical. Biomembrane is the main place to undertake lipid peroxidation injury. Active oxygen from inflammatory cells could induce apoptosis in cells when lipid peroxidation injury occurs to the membranes of mitochondria and microsome. Changes of cell phenotype through gene transcription might be one of the mechanisms of apoptosis induced by oxidation injury. The present study also suggested that picroside II could simultaneously decrease the level of MDA, ALT, AST, and increase the activity of SOD in liver mitochondria.
In conclusion, we have shown for the first time that picroside II is able to protect hepatocytes against injury and counteract apoptosis through its anti-oxidation effect, and it can act by decreasing MDA level, increasing the activity of SOD in liver mitochondria and upregulating bcl-2 gene expression.
References
- Ansari RA, Aswal BS, Chander R, Dhawan BN, Garg NK, Kapoor NK, et al. Hepatoprotective activity of kutkin, the iridoid glycoside mixture of Picrorhiza kurroa. Indian J Med Res 1998;87:401-4.
- Chander R, Wivedi Y, Rastogi R, Sharma SK, Garg NK, Kapoor NK, et al. Evaluation of hepatoprotective activity of Picroliv (from Picrohiza kurroa) in Mastomys natalensis infected with Plasmodium berghei. Indian J Med Res 1990;92:34-7.
- Dwivedi Y, Rastoui R, Chander R, Sharma SK, Garg NK, Dhawan BN. Hepatoprotective activity of Picroliv against carbon tetrachloride induced damage in rats. Indian J Med Res 1990;92:195-200.
- Saksena S, Rastogi R, Garg NK, Dhawan BN. Rifampicin induced hepatotoxicity in rats: protective effect of Picroliv. Drug Dev Res 1994;33:46-50.
- Saraswat B, Visen P KS, Patnaik GK, Dhawan BN. Hepatopro-tective activity of Picroliv against rifampicin induced toxicity. Drug Dev Res 1997;40:299-303.
- Marland S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogollol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47:469-74.
- Lowry OH, Rosebrough NJ, Farr AL. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
- Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med 1976;15:212-5.
- Guo JT, Zhou H, Liu C, Aldrich C, Saputelli J, Whitaker T, et al. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J Virol 2000;74:1495-505.
- Honda A, Hatano M, Kohara M, Arai Y, Hartatik T, Moriyama T, et al. HCV-core protein accelerates recovery from the insensitivity of liver cells to Fas-mediated apoptosis induced by an injection of anti-Fas antibody in mice. J Hepatol 2000;33:440-7.
- Gu XH, Li QF, Wang YM. Expression of bcl-2, bax and hepatocyte apoptosis in the liver tissues of hepatitis D patients. Chin J Hepatol 2001;9:81-3.
- Williams GT, Smith CA. Molecular regulation of apoptosis: genetic controls on cell death. Cell 1993;74:777.
- Tokushige K, Yamaguchi N, Ikeda I, Hashimoto E, Yamauchi K, Hayashi N. Significance of soluble TNF receptor-1 in acute-type fulminant hepatitis. Am J Gastroenterol 2000;95:2040-6.
- Ryo K, Kamogawa Y, Ikeda I, Yamauchi K, Yonehara S, Nagata S, et al. Significance of Fas antigen-mediated apoptosis in human fulminant hepatic failure. Am J Gastroenterol 2000;95:2047-55.
- Van Molle W, Berghe JV, Brouckaert P, Libert C. Tumor necrosis factor-induced lethal hepatitis: pharmacological intervention with verapamil, tannic acid, picotamide and K76COOH. FEBS Lett 2000;467:201-5.
- Okamoto T. The protective effect of glycyrrhizin on anti-Fas antibody-induced hepatitis in mice. Eur J Pharmacol 2000;387:229-32.
- Fiorucci S, Mencarelli A, Palazzetti B, Soldato PD, Morelli A, Ignarro LI. An NO derivative of ursodeoxycholic acid protects against Fas-mediated liver injury by inhibiting caspase activity. Proc Natl Acad Sci USA 2001;98:2652-7.
- Yamamoto M, Miura N, Ohtake N, Amagaya S, Ishige A, Sasaki H, et al. Genipin, a metabolite derived from the herbal medicine Inchin-ko-to, and suppression of Fas-induced lethal liver apoptosis in mice. Gastroenterology 2000;118:380-9.
- Liu J, Shen HM, Ong CN. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG(2) cells. Cancer Lett 2000;153:85-93.
- Shimizu I. Sho-saiko-to: Japanese herbal medicine for protection against hepatic fibrosis and carcinoma. J Gastroenterol Hepatol 2000;15:D84-90.
- Amato D, Zwain I, Reed J. Expression of bcl-2 and bax in human placenta. J Soc Gynecol Invest 1996;3 Suppl:226.
- Zhang GQ, Yu H, Zhou XQ, Liao D, Xie Q, Wang B. TNF-α induced apoptosis and necrosis of mice hepatocytes. World Chin J Diges 2000;8:303-6.
- Xu Y, Blalik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, et al. NF-κB inactivation converts a hepatocyte cell line TNF-α response from proliferation to apoptosis. Am J Physiol 1998;275:C1058-66.
- Leist M, Gantner F, Bohlinger I. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-α requires transcriptional arrest. J Immunol 1994;153:1778-88.
