Protective effects of bendazac lysine on early experimental diabetic nephropathy in rats
Introduction
Diabetic nephropathy (DN) is now a leading cause of end-stage renal failure in many countries. More than 30% of diabetes mellitus patients develop clinically evident DN 10 to 20 years after the onset of diabetes mellitus. DN seems to occur as a result of interaction of metabolic and hemodynamic factors. Several biochemical pathways, such as increased oxidative stress and advanced glycation end-products (AGE)[1,2], hyperactivity of aldose reductase (AR) in the polyol pathway[3], and hyperactivity of pro-sclerotic cytokine, such as transforming growth factor-β1 (TGF-β1)[4] and connective tissue growth factor (CTGF)[5], have been proposed as candidates for explaining the mechanism of the progressive cause of DN. For the complexity of mechanism, there is still no definitive therapy halting the development of DN that afflicts diabetic patients. Therefore, it is necessary to develop a novel therapy strategy for DN that can deal with more than one pharmacological target in this intricate mechanism.
Bendazac lysine (BDL) is one of agents that have been introduced for the management of cataracts, protecting the level of vision in patients, thus delaying the need for surgical intervention. Its principal effects are to inhibit the denaturation of proteins and AR activity, responsible for the accumulation of sorbitol and water retention in the lens fibers[6]. Several studies show that BDL has an inhibitive effect on AR[7], a main enzyme in the polyol pathway, an antioxidative effect[8], and an inhibitive effect on glycosylation[9], all of which are included in the progression of DN. Since there are still very few published reports focused on the effects of BDL on DN. Therefore, it is worthwhile for us to explore its potential effects on preventing the progression of DN.
To evaluate the effects of BDL on DN, we studied the possible influence of BDL on the parameters that indicate protective effects against the progress of DN, such as blood glucose level, AR activity, AGE level, laminin level in the kidney cortex, thickness of the glomerular base membrane (GBM) and expression of TGF-β1 mRNA in the kidney cortex on early experimental DN rats, and observe their morphological changes.
Materials and methods
Drugs BDL was synthesized by Supereyes (Lot N
Animals Male Sprague-Dawley rats (Grade I, Certificate N
Induction of DN model and study protocol Diabetic rats were induced with an ip injection of 60 mg/kg of STZ (dissolved in pH 4.5 citrate buffer immediately before injection), while controlled normal standard rats (NS group, n=10) received 2.5 mL/kg of citrate buffer. Induction of the diabetic state was confirmed by measuring the blood glucose level at the 72 h after the injection of STZ. The rats whose blood glucose concentrations were ≥13.88 mmol/L were randomly allotted into 5 groups: DN rats treated with 1% CMC solution (DN group, n=10); DN rats treated with 100, 200, and 400 mg/kg of BDL for BL group (low dose, n=10), BM group (moderate dose, n=11), and BH group (high dose, n=10), respectively; and DN rats treated with 100 mg/kg of epalrestat (EPS group, n=10). The same volume of CMC solution was administered to the NS group (n=10). The animals were housed in a controlled environment (24±1 °C, 12-h light: 12-h dark cycle, onset of light at 07:00 AM) and were allowed food and water ad libitum. After 8 weeks, urine and blood samples were collected. After animals were sacrificed, fresh kidney cortices were stored in formaldehyde solution for light microscopic observation, and 1 mm×1 mm×1 mm cubes of kidney cortices were fixed in 2.5% glutaraldehyde for electron microscopic measurement. The rest of the kidneys were stored at -75 °C for the later analysis.
Measurement of renal function and biochemical parameters Kidney index was the ratio of kidney weight versus body weight. Blood glucose was measured by the glucose oxidase method with kits purchased from Dong-Ou Bioengineering (N
To analyze the total antioxidative capability, 2.0 mL of ABTS [2,2'-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid]/myoglobin reagent was mixed with 20 µL of sample and a further 180 µL of diluent, which flushed the sample probe. The initiator of the reaction, hydrogen peroxide (0.675 mmol/L, 250 µL), was added last to get a final concentration in the cuvette of 75 µmol/L. After a 6-min incubation, the optical density (OD) of ABTS•+ was read at 734 nm by spectrophotometer (UV-1600, Beijing, China), and the value of total antioxidative capability was calculated[10].
The AR activity was measured by fluorospectrophoto-meter (Shimadzu, RF-5300, Kyoto, Japan) with reagents of β-NADPH (Lot N
Laminin, a main component of the extracellular matrix, was determined by the radioimmunoassay method, using kits from Shanghai High Biotech Center (N
RT-PCR for the relative quantity of TGF-β1 mRNA in kidney cortex RT- PCR was performed to determine the relative quantity of TGF-β1 mRNA in the kidney cortex, whereas β-actin mRNA, a housekeeping gene, was used as an internal control[12]. Briefly, total RNA was extracted from the kidney cortex with TRIzol (Lot N
Morphological observation and measurement of thickness of GBM Kidney cortex samples stored in formaldehyde solution were embedded with paraffin and stained with HE. Each HE-stained sample in each group was observed under light microscope. Three kidney samples from each experimental group were randomly chosen for electron microscopic observation. Specimens were embedded in epoxy resin and cut into ultrathin sections and then stained with plumbum citrate for ultrastructural observation under transmission electron microscope (JEM 1200EX, Jeol, Tokyo, Japan). Five photos were taken at different views for each kidney sample. The images were amplified 10K and the photos were scanned into a computer to measure the thickness of GBM using an image analysis system (Leica Qwin Standard V2.6, Leica Microsystems, Welzlar, Germany).
Statistical analyses Statistical analysis was performed to compare the effects of BDL on early DN rats using one-way analysis of variance (ANOVA) and Dunnett’s t-test (2-side) for the different groups using SPSS 10.0. Data were expressed as mean±SD. P<0.05 was considered statistically significant.
Results
Effects of BDL on physical behaviors, blood glucose, microalbuminuria, and kidney index In our experiment, rats in the DN group displayed the following physical characteristics: hypopraxia, cachexia, yellowish and damp fur, kyphosis, body shake, ptosis, polyuria polydipsia and tardy weight gain; while rats in NS and BH groups were vibrant, vigorous, with white and tidy fur, and weight gain.
The blood glucose level, microalbuminuria, and kidney index of the DN group were significantly higher than those of NS group (P<0.01), indicating that our early DN model was successful. Low doses of BDL slightly reduced blood glucose in DN rats, but greatly reduced microalbuminuria (P<0.01). High doses of BDL significantly reduced blood glucose and microalbuminuria level in DN rats (P<0.01). BDL (low, moderate and high doses) and epalrestat slightly reduced the kidney index, but differences were not significant (Table 1).
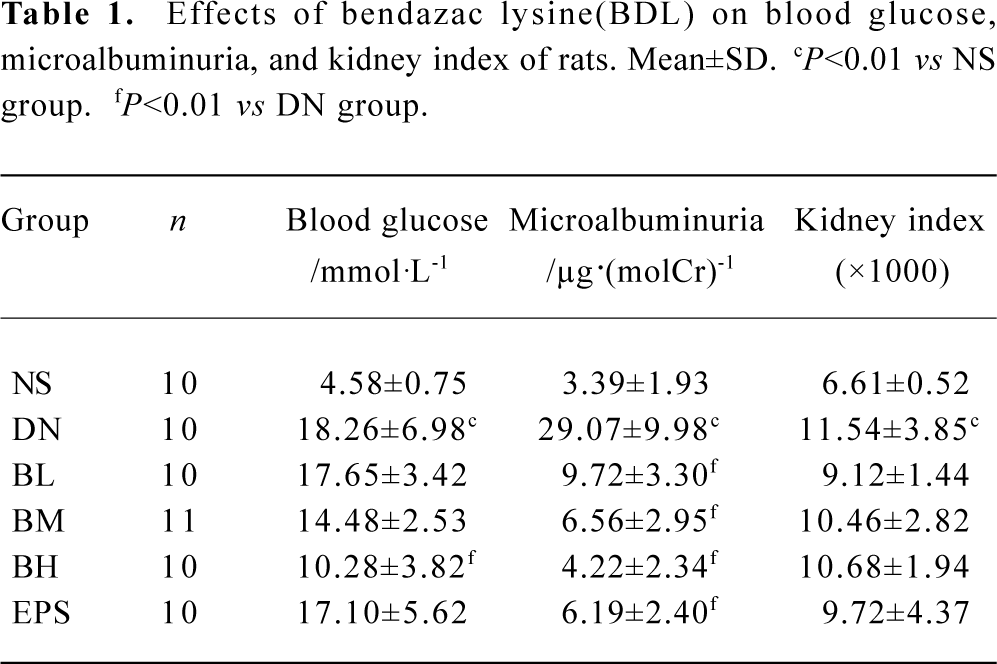
Full table
Effects of BDL on laminin level in kidney cortex and thickness of GBM The level of laminin in the kidney cortex of early DN rats significantly increased, when compared with that of normal rats (P<0.01). Three doses of BDL caused a decrease in the level of laminin, but a greatly significant decrease can be found only in high doses (P<0.01) (Figure 1A).

There was a significant difference in the thickness of GBM between the NS group and DN group (P<0.01). The thickness of GBM decreased with increasing doses of BDL. Compared with the DN group, there were significant differences in the thickness of GBM of BL, BM, BH and EPS groups (P<0.05, or P<0.01) (Figure 1B).
Effect of BDL on AR activity in erythrocyte AR activities in the erythrocyte of NS and DN groups were 6.90 and 27.29 U, respectively (P<0.01). Significant differences of AR activities at low, moderate, and high dose BDL-treated rats existed when compared with CMC-treated DN rats (P<0.01). Furthermore, AR activity and BDL doses showed good dose-dependence. Epalrestat, an AR inhibitor, also significantly reduced AR activity from 27.29 to 11.07 U, showing a very significant difference (P<0.01) (Figure 2).
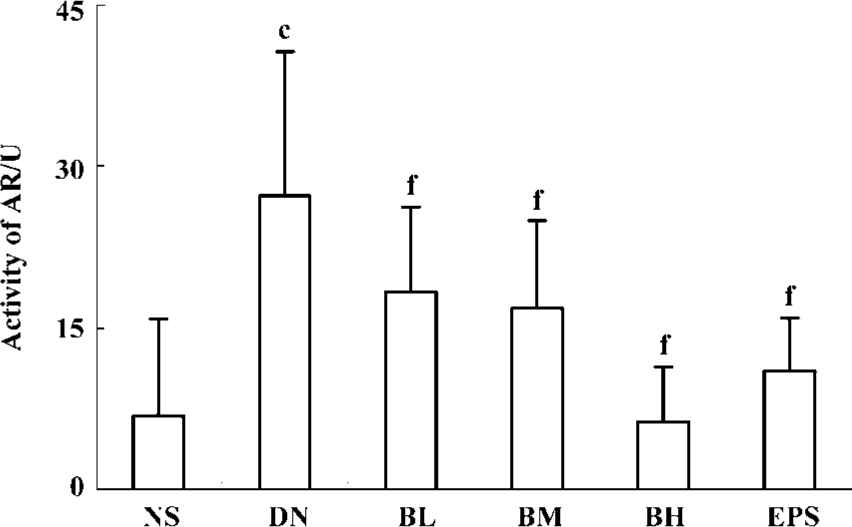
Effects of BDL on AGE and oxidative stress AGE levels in the kidney cortex and in the serum of DN rats were greatly higher than those of normal rats (P<0.01). Both in the kidney cortex and in serum, there were significant decreases in AGE levels for BL, BM, and BH groups when compared with the DN group (P<0.05, or P<0.01), whereas epalrestat had the same significant difference (P<0.01). In contrast, the total antioxidative capability in serum, which expresses the anti-oxidative status of animals, was reduced significantly in DN rats. Three doses of BDL and epalrestat significantly enhanced the total antioxidative capability of DN rats (P<0.01) (Table 2).

Full table
Effect of BDL on the relative quantity of TGF-β1 mRNA in kidney cortex After the RT-PCR procedure, the amplified products of TGF-β1 mRNA were completely separated by electrophoresis (Figure 3A). The relative quantity of TGF-β1 mRNA in the kidney cortex of the DN group significantly increased when compared with that of NS group (P<0.01). Low, moderate, and high dose BDL caused the level of TGF-β1 mRNA to decrease, and statistical differences existed when compared with CMC-treated DN rats (P<0.01). Epalrestat had the same effect as a moderate dose of BDL (P<0.01) (Figure 3B).
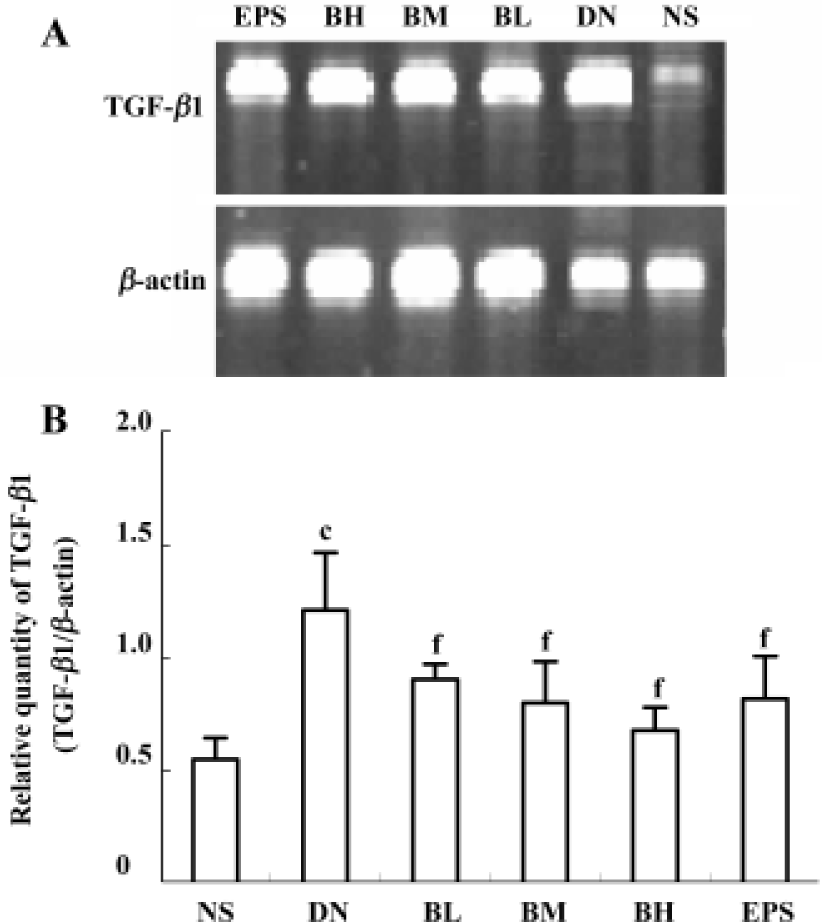
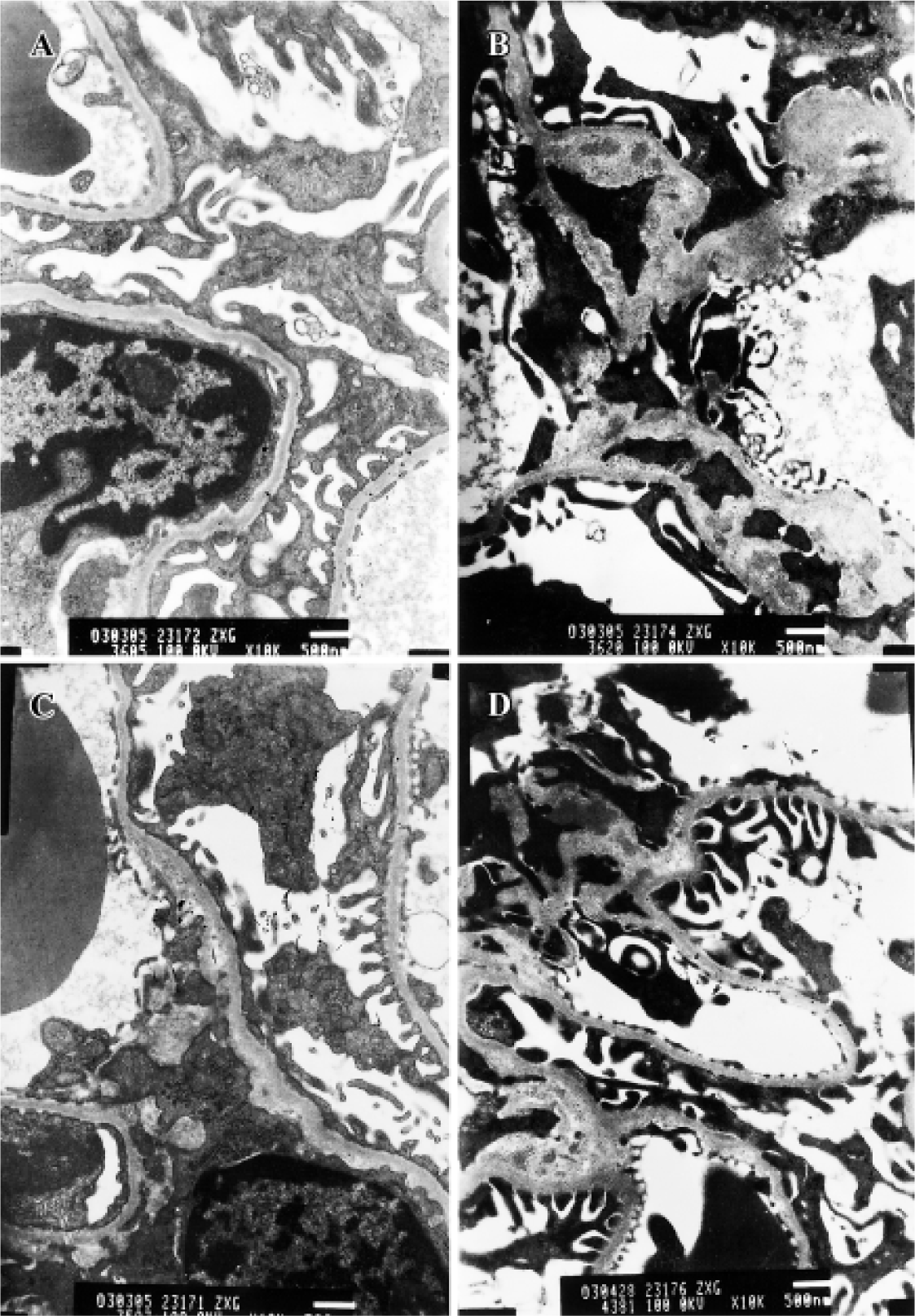
Effects of BDL on morphological change in kidney The light microphotograph showed that glomerular mesangial hyperplasia existed (Figure 4). In the transmission electron micrographs, the ultrastructure of glomerulus of the CMC-treated DN rat was changed. The GBM was wrinkled and partly thickened, with effacement of some visceral epithelial cell foot processes and microvillus transformation. After 8 weeks of treatment with 400 mg/kg of BDL, the glomerular capillary loops, GBM, pedicelsa and mesangial matrix of rats appeared to be almost normal (Figure 5).
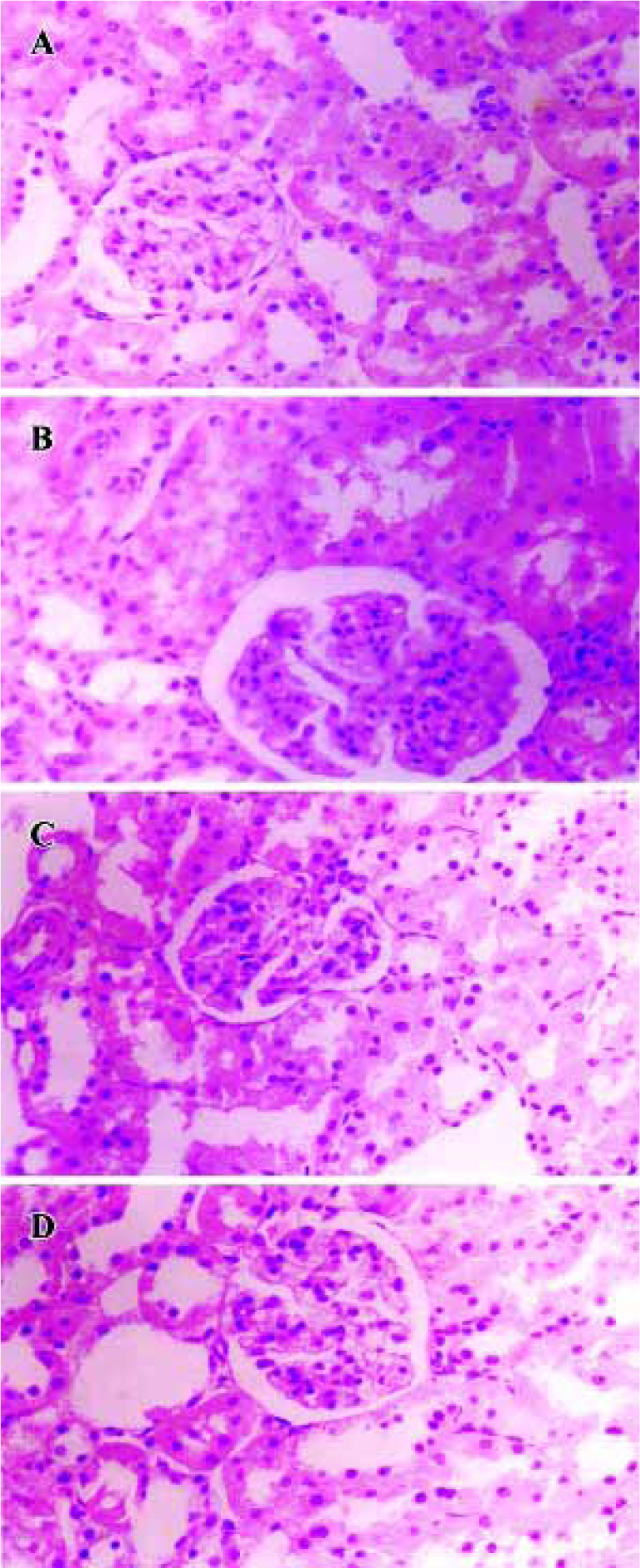
Discussion
Oxidative stress plays an important role in the etiology of diabetic complications[13], and the formation of reactive oxygen species is a direct consequence of hyperglycemia. In our study, we found that the total antioxidative capability (a common indicator for changes in the antioxidation system), which includes ascorbate, protein thiols, bilirubin, urate and α-tocopherol, was significantly increased by BDL, strongly suggesting that BDL has an effect on antioxidative capability in vivo. This result is consistent with a former report, in which BDL inhibited in vitro depolymerisation of hyaluronic acid by free hydroxyl and superoxide radicals from a sodium ascorbate system[14]. Furthermore, we also found that BDL, even in low doses, significantly decreased AGE levels either in the kidney cortex or in serum. Thus, the antioxidative effect of BDL is very important and can be of great clinical significance for DN.
The status of oxidative stress in intensity and durability facilitates the formation of AGE, which is the result of a reaction between carbohydrates and free amino group of proteins. AGE accumulates in extracellular matrix proteins as a physiological process during ageing. However, this accumulation happens earlier, and with an accelerated rate, in diabetes mellitus than in non-diabetic individuals[15]. AGE also enhances the susceptibility of LDL to oxidation[16] and induces apoptosis in cultured human umbilical vein endothelial cells[17]. In reverse, the interaction between AGE and their specific receptor (RAGE) induces the activation of oxidative stress, and stimulates the production and release of cytokines, amplifying the tissue damage[2]. It can be concluded that oxidative stress and AGE interact with and upregulate each other. As already stated, the major metabolite of bendazac inhibited glycosylation by sugar in a dose-dependent manner[18], and also bendazac and its metabolite (5-hydroxybendazac) inhibit the carbamylation of soluble lens proteins in vitro[19]. Furthermore, in our study, BDL significantly decreased AGE levels both in the kidney cortex and in serum. These data strongly suggest the anti-AGE character of BDL and this could be of benefit for the prevention of DN.
Our data shows that BDL has an inhibitory effect on the relative quantity of TGF-β1 mRNA in the kidney cortex of DN rats, which is commonly considered as a pivotal cytokine in mediating the progression of DN. In vitro, studies have shown that a range of stimuli increase TGF-β1 expression, such as hyperglycemia, AGE, and various products of oxidative stress[20,21]. Indeed, TGF-β1 seems to be an important site of interaction between hemodynamic and metabolic pathways, playing a key role in the synergy between hypertension and hyperglycemia in mediating DN[22]. Therefore, having an inhibitory effect on TGF-β1, BDL affects the regulation of several cytokine in the progression of DN, and is a prime candidate for developing appropriate agents in the prevention and treatment of DN.
Laminin, kidney index and the thickness of GBM are usually enlarged during the progression of DN and serve as persuasive parameters for describing glomerular hyper-trophy. Although a decrease in the kidney index for 3 doses of BDL were not defined significantly in our experiment, the thickness of GBM, another significantly decreased morphological parameter in our study, together with laminin, which describes the level of extracellular matrix, still suggest that BDL can aid in preventing renal hypertrophy.
The accumulation of polyols in the kidney has been suggested to be involved in the development of DN. In diabetic patients, AR activity is greatly enhanced by high glucose and accumulated sorbitol, which is associated with the depletion of myoinositol and changes in the cellular redox potential[23], resulting in deteriorative function in nephropathy, retinopathy, and neuropathy. The importance of AR relevant to DN has also been emphasized with the demonstration that the increase of AR activity is associated with enhanced protein kinase C activation and TGF-β1 production in human mesangial cells in response to glucose[24]. In experimental diabetes, several studies have examined the effects of AR inhibition on various functional and structural markers of DN. In an in vitro experiment, the AR inhibitor, epalrestat, effectively corrected glucose-induced imbalances of the polyol pathway and myo-inositol uptake in neutrophils and was capable of ameliorating the neutrophil dysfunction[25]. Also, epalrestat lowers the level of Nepsilon-(carboxymethyl)lysine (CML) and associated variables, and polyol metabolites are correlated with CML in the erythrocytes of diabetic patients[26]. In a clinical study, the effects of epalrestat on autonomic and somatic neuropathy were assessed, suggesting that epalrestat has therapeutic value at early stages of DN[27]. In our current experiment, we found that BDL significantly lowered AR activities in rats from 27.29 U to 18.38 U (low dose), 16.92 U (moderate dose), and 6.39 U (high dose), while epalrestat lowered AR activities to 11.07 U (100 mg/kg). The effect of high doses of BDL on AR activity was stronger than that of a common dose of typical AR inhibitor, epalrestat, and therefore, testifying that BDL is a strong inhibitor for AR. Hence, besides its recognized cataract delaying effect on the communal pathway in the pathology of cataracts, BDL can be justified in its clinical application for DN treatment.
BDL is an oxyacetic acid with protein antidenaturant, anti-inflammatory, antinecrotic, choleretic and antilipidemic properties. In vitro, BDL inhibited the carbamylation of soluble lens proteins, and the depolymerisation of hyaluronic acid by free hydroxyl and superoxide radicals from a sodium ascorbate system[14]. Bendazac also has scavenger-like activity as a result of its interaction with protein molecules[6]. In our experiment, BDL and epalrestat (a commonly used DN-treatment agent) ameliorated the physical morbidity, reduced microalbuminuria, laminin level, thickness of GBM, AGE level, total antioxidative capability, AR activity, and TGF-β1 mRNA levels in DN rats. What must be pointed out is that BDL significantly decreased blood glucose and ameliorated the persuasive pathological changes by light and electron micrograph, while epalrestat has no effect on them. In the complicated mechanisms of DN development, the pathway of hyperglycemia-oxidative stress-AGE/TGF-β1 is assuredly very important. Many renal changes are the consequences of changes in blood glucose levels, and many ameliorative effects of BDL on DN may be closely dependant on its effect on the blood glucose level. Therefore, compared with epalrestat, BDL might have more widely therapeutic points for DN.
To sum up, in our STZ induced early DN rat model, BDL decreased the blood glucose level, reduced the level of AGE, the intensity of oxidative stress, AR activity, and the relative level of TGF-β1 mRNA and, furthermore, lowered the laminin level in the kidney cortex and decreased the thickness of GBM, and therefore ameliorated the morbidity in physical behavior, morphology, and microalbuminuria. In conclusion, BDL has protective effects on several pharmacological targets in the complicated pathology mechanism of DN. It is therefore worthwhile to study further its potential protective effects on early DN.
References
- Catherwood MA, Powell LA, Anderson P, McMaster D, Sharpe PC, Trimble ER. Glucose-induced oxidative stress in mesangial cells. Kidney Int 2002;61:599-608.
- Wautier JL, Guillausseau PJ. Advanced glycation end products, their receptors and diabetic angiopathy. Diabetes Metab 2001;27:535-42.
- Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia 2001;44:1957-72.
- Sharma K, McGowan Tracy A. TGF-β in diabetic kidney: role of novel signaling pathways. Cytokine Growth Factor Rev 2000;11:115-23.
- Liu BC, Chen Q, Luo DD, Sun J, Phillips AO, Ruan XZ, et al. Mechanisms of irbesartan in prevention of renal lesion in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 2003;24:67-73.
- Balfour JA, Clissold SP. Bendazac lysine: a review of its pharmacological properties and therapeutic potential in the management of cataracts. Drugs 1990;39:575-96.
- Inliano G, Menzion M, Apponi-Battini G, Costagliola C. Inhibition of rat lens aldose reductase by bandazac-L-lysine salt. Enzyme 1989;42:235-7.
- Schmut O, Hofmann H. Studies on the activity of bendalina (bendazac L-lysine salt) as a scavenger of hydroxyl and superoxide radials. Wien Klin Wochenschr 1985;97:819-22.
- Marques C, Ramalho JS, Pereira P, Mota MC. Bendazac decreases in vitro glycation of human lens crystallins. Decrease of in vitro protein glycation by bendazac. Doc Ophthalmol 1995;90:395-404.
- Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 1993;84:407-12.
- Das B, Srivastava SK. Purification and properties of aldose reductase and aldehyde reductase II from human erythrocyte. Arch Biochem Biophy 1985;238:670-9.
- Kaneto H, Morrissey J, Klahr S. Increased expression of TGF-β1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int 1993;44:313-21.
- Ceriello A, Giugliano D, Quatraro A, Dello Russo P, Lefebvre PJ. Metabolic control may influence the increased superoxide generation in diabetic serum. Diabetic Med 1991;8:540-2.
- Schmut, Fellinger C, Hofmann H. Bendazac lysine: a protective substance against oxygen-free radicals. In: D’Ermo F. Recent developments in the pharmacological treatment of cataract. Amsterdam: Kugler Publications; 1987. p 69–72.
- Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product Nε-(carboxymethyl) lysine in human tissues in diabetes and aging. J Clin Invest 1997;99:457-68.
- Ihm SH, Yoo HJ, Park SW, Ihm J. Effect of aminiguanidine on lipid peroxidation in streptozotocin-induced diabetic rats. Metabolism 1999;48:1141-5.
- Min C, Kang E, Yu SK, Shinn SH, Kim YS. Advanced glycation end products induce apoptosis and procoagulant activity in cultured human umbilical vein endothelial cells. Diab Res Clin Pract 1999;46:197-202.
- Lewis BS, Harding JJ. The major metabolite of bendazac inhibits the glycosylation of soluble lens proteins: a possible mechanism for a delay in cataractogenesis. Exp Eye Res 1988;47:217-25.
- Lewis BS, Rixon KC, Harding JJ. Bendazac prevents cyanate binging to soluble lens proteins and cyanate-induced phase-separation opacities in vitro: a possible mechanism by which bendazac could delay cataract. Exp Eye Res 1986;43:973-9.
- Jandeleit-Dahm K, Cao ZM, Cox AJ, Kelly DJ, Gilbert RE, Cooper ME. Role of hyperlipidemia in progressive renal disease: focus on diabetic nephropathy. Kidney Int 1999;56 Suppl 71:S31-6.
- Montero A, Munger KA, Khan RZ, Valdivielso JM, Morrow JD, Guasch A, et al. F-2-isoprostanes mediate high glucose-induced TGF-beta synthesis and glomerular proteinuria in experimental type I diabetes. Kidney Int 2000;58:1963-72.
- Yin XX, Zhang YD, Wu HW, Zhu X, Zheng XG, Jiang SJ, et al. Protective effects of Astragalus Sapoonin I on early stage of diabetic nephropathy in rats. J Pharmacol Sci 2004;95:256-66.
- Greene DA, Lattimer SA, Sima AAF. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complicatins. N Engl J Med 1987;316:599-606.
- Ishii H, Tada H, Isogai S. An aldose reductase inhibitor prevents glucose-induced increase in transforming growth factor-beta and protein kinase C activity in cultured human mesangial cells. Diabetologia 1998;41:362-4.
- Suzuki K, Kawamura T, Sakakibara F, Sasaki H, Sano T, Sakamoto N, et al. Effect of aldose reductase inhibitors on glucose-induced changes in sorbitol and myo-inositol metabolism in human neutrophils. Diabet Med 1999;16:67-73.
- Hamada Y, Nakamura J, Naruse K, Komori T, Kato K, Kasuya Y, et al. Epalrestat, an aldose reductase inhibitor, reduces the levels of Nepsilon-(carboxymethyl)lysine protein adducts and their precursors in erythrocytes from diabetic patients. Diabetes Care 2000;23:1539-44.
- Nakayama M, Nakamura J, Hamada Y, Chaya S, Mizubayashi R, Yasuda Y, et al. Aldose reductase inhibition ameliorates papillary light reflex and F-wave latency in patients with mild diabetic neuropathy. Diabetes Care 2001;24:1093-8.
