Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotype in healthy Chinese subjects1
Introduction
S-Mephenytoin-4’-hydroxylase (CYP2C19) is a genetically determined enzyme and its phenotypes can be classified as poor metabolizer (PM) and extensive metabolizer (EM)[1,2]. When CYP2C19 is the main metabolism enzyme of a drug, the pharmacokinetics of the drug are different between the PM and EM phenotypes, such as proton pump inhibitors (PPI). Recently, rabeprazole (RPZ) has been reported to be metabolized mainly via a non-enzymatic pathway, with only minor CYP2C19 and CYP3A4 involvement[3–6]. The pharmacokinetics of RPZ are assumed to be less influenced by the CYP2C19 phenotype. The incidence of PM for CYP2C19 in the Chinese population is very high (17.4%)[7]. However, it is not clear whether the pharmacokinetics and pharmacodynamics of RPZ depend on the CYP2C19 genotype status in Chinese people. Thus, studies examining the effects of CYP2C19 genetic polymorphism on the metabolism of RPZ in Chinese people are important. In the present study we observed the metabolic disposition characteristics and pharmacodynamics of RPZ after a single dose and after 8 days of repeated doses with reference to different CYP2C19 genotype groups to provide valuable data that should be considered when selecting PPI for patients with acid-related diseases with reference to the CYP2C19 genotype status.
Materials and methods
Subjects and CYP2C19 genotypes Helicobacter pylori (H pylori) infection was screened using a serological test (Dot-immunogold kit, Lanbo Bio-Tech Institute, China) and a 13C-urea breath test. DNA was extracted from each indivi-dual’s leucocytes using a commercially available kit (Promega, Madison, WI, USA). Genotyping procedures for identifying the CYP2C19 wild-type (CYP2C19*1) and the two mutated alleles, CYP2C19*2 and CYP2C19*3, were carried out using the polymerase chain reaction and restriction fragment length polymorphism method[8].
A total of 20 H pylori-negative healthy volunteers participated in this study. Seven subjects were classified as homozygous extensive metabolizers (homEM). Six were heterozygous for exon 5 mutation of CYP2C19 (*1/*2) or heterozygous for the exon 4 mutation (*1/*3) and were classified as heterozygous extensive metabolizers (hetEM). The remaining seven subjects were homozygous for the exon 5 mutation (*2/*2) and were classified as the PM group (Table 1).
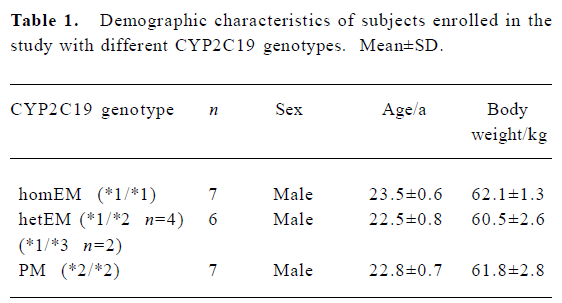
Full table
None of the subjects consumed alcohol or smoking. None of the subjects had taken any drugs for at least 4 weeks before or during the study. The Ethics Committee of Anhui Medical University approved the protocol in advance. Written informed consent was obtained from each subject before participation in the study.
Study protocol All healthy volunteers were orally treated with 20 mg RPZ (Pariet, Eisai Company, Tokyo, Japan) for an 8-day period. The medication was taken once daily at 8:00. The 24-h intragastric pH monitoring and the measurement of serum levels of RPZ were carried out on day 1 and day 8. Two standard meals (12:00, 18:00), prepared at the hospital, were provided for each subject.
Intragastric pH measurement After overnight fasting, a glass electrode was inserted transnasally and placed approximately 5 cm below the cardia. The electrode was calibrated with standard buffers (pH 1.07 and 7.01) before recording the pH with a Digitrapper pH (Medtronic, Watford,UK). Intragastric pH recordings started after the oral dose of RPZ at 8:00 on d 1 and d 8.
Sample collection and concentration assays of rabepra-zole Blood samples were collected before and 0.5, 1, 1.5, 2, 3, 5, 7, 10, 12, and 24 h after RPZ administration on day 1 and day 8. After collection, the blood samples were immediately centrifuged at 4000 r/min for 10 min and 100 µL of 1% diethylamine solution was added to the 1 mL sample of RPZ plasma. All samples were stored at –80°C until assayed. Plasma levels of RPZ were measured using high performance liquid chromatography[9,10] . The lower detection limit for RPZ was 0.01 mg/L. A good linearity is obtained from 0.01–0.75 mg/L of RPZ with r=0.999. The standard curve of RPZ in serum is Y=124950X – 806.05 (n=5). The recoveries of three concentra-tions, 0.05, 0.1, 0.5 mg/L are 75.2%, 84.2%, and 91.0%, respectively. The RSD of intra-day variation of RPZ are 5.1%, 9.2%, and 6.8% and the RSD of inter-day variation are 8.2%, 3.5%, and 4.2% for the three concentrations, respectively.
Statistical analysis Intragastric pH characters were described by the median, mean, pH>4 total time and the pH>4 time proportion of 24 h from the raw pH values. The values for the areas under the serum concentration-time curves (AUC) from 0 to 24 h for RPZ were calculated using the 3P87 software. All P values are two-sided, and P<0.05 indicated statistical significance. Data were expressed as mean±SD. Statistically significant differences in the mean AUC values for RPZ and intragastric pH values between the three different CYP2C19 genotype groups were compared using a one-way analysis of variance (ANOVA) combined with the least significant method (LSD). Paired t-tests were used to determine whether there were differences in the AUC values and intragastric pH values for RPZ between single and repeated doses. Statistical calculations were carried out using SPSS 11.0 software (SPSS Inc, Chicago, USA).
Results
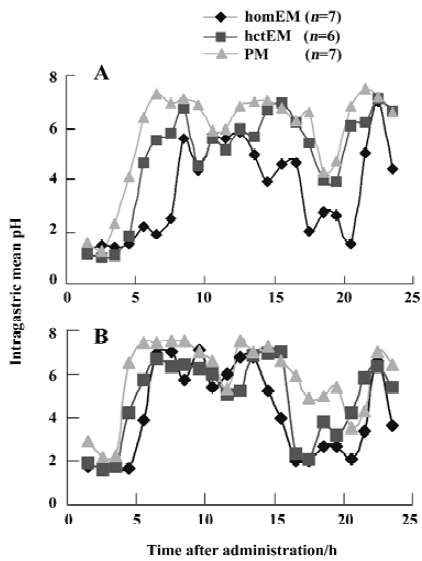
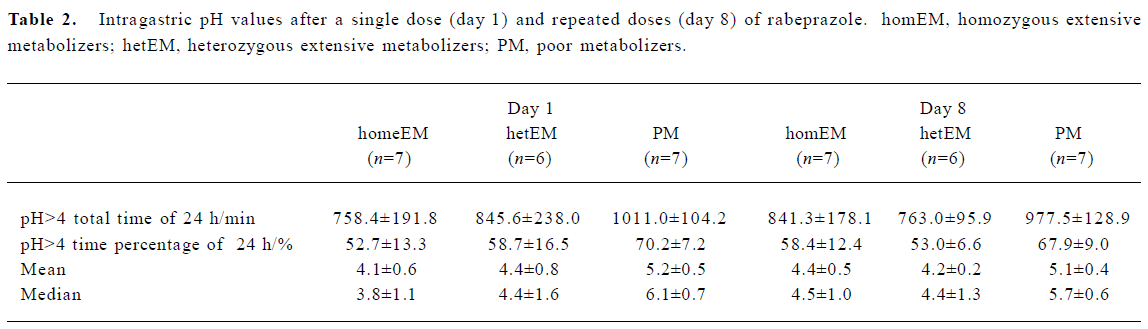
Role of the CYP2C19 genotype on the acid-inhibitory efficacy of rabeprazole Raw data on the mean intragastric pH-time curves after single and repeated doses of RPZ in the three different genotype groups are shown in Figure 1. The characteristic values of the 24-h intragastric pH after single and repeated doses of RPZ in the three different genotype groups are summarized in Table 2. The median intragastric pH value of the PM group was the highest, followed by the hetEM group, and the homEM group had the lowest value.
Full table
No significant differences in intragastric pH values were observed between the three groups after a single dose or after repeated doses for 8 days of RPZ. In addition, no significant increments in intragastric pH values from single to repeated doses were observed in the three different genotype groups.
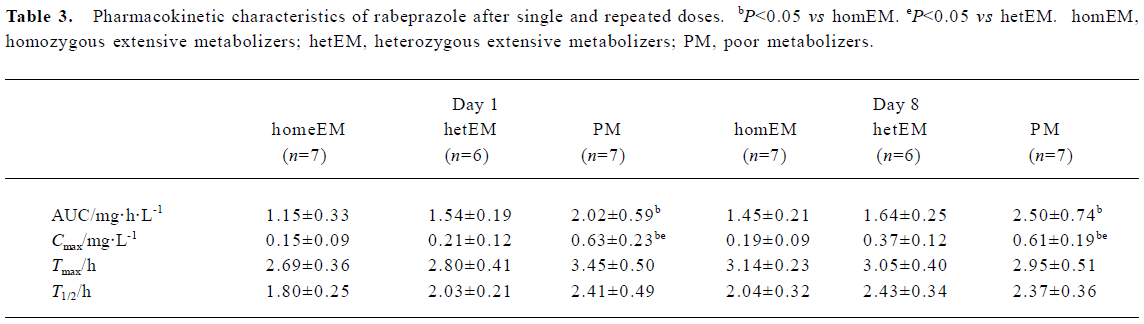
Role of the CYP2C19 genotype on the kinetic disposition of rabeprazole The pharmacokinetic parameters are shown in Table 3. The mean AUC values for RPZ after a single dose differed among the three different genotype groups, with a relative ratio of 1.0, 1.3, and 1.8 in the homEM, hetEM and PM groups, respectively. The mean AUC values for RPZ after repeated doses also differed among the three groups, with a relative ratio of 1.0, 1.1, and 1.7 in the homEM, hetEM and PM groups, respectively. The mean AUC values for RPZ after single and repeated doses were significantly different between the homEM and PM groups, but not between the homEM and hetEM or between the hetEM and PM groups. No significant increase in the mean AUC values for RPZ from single to repeated doses was observed in any of the three different genotype groups. The Cmax values were significantly different between the homEM and PM groups, and the hetEM and PM groups after single and repeated doses of RPZ. Whereas Tmax and T1/2 did not differ significantly between the three groups on day 1 and day 8.
Full table
Discussion
Proton pump inhibitors, such as omeprazole, lansopra-zole, pantoprazole, and rabeprazole, have been used widely in the treatment of acid-related diseases. Recent research has paid more attention to the inhibitory effects of PPI in relation to the genetic polymorphism of CYP2C19, a major enzyme for the metabolism of PPI in the liver[11]. These studies have shown that CYP2C19 genetic polymorphism has a significant influence on acid-inhibitory efficacy and the metabolism of omeprazole in healthy Chinese Han subjects. However, to date no studies examining the relationship between the CYP2C19 genotype and the metabolism of RPZ has been carried out in the Chinese Han population. In vitro human liver microsomal and in vivo human pharmacology studies have shown that RPZ is metabolized mainly via a non-enzymatic reduction to RPZ thioether, and that CYP2C19 and CYP3A4 are partially involved in the metabolism of RPZ[3-6]. The existence of CYP3A4-related PM has not been reported in any Chinese population. Therefore, we did take into account CYP2C19-related genotyping factors in our study.
Rabeprazole has a rapid and powerful onset of pharmacological action[12]. Our study showed that the AUC for RPZ after a single dose exceeded 80% of the AUC after repeated doses and there were no significant increments from single to repeated doses, which was consistent with Yasuda et al[9]. In addition, we found that no significant increment in intragastric pH values was observed from single to repeated doses. These results suggest that the metabolism of RPZ after a single dose could attain maximum acid-inhibitory efficacy. And this appears to be the reason for pH values remaining elevated for more than 50% of the time, even with very modest exposures, and when the pharmacokinetic results on day 1 are consistent with the results on day 8.
Adachi and other researchers[3-6] have found that the acid-inhibitory efficacy and metabolism of RPZ are not dependent on CYP2C19 genotype status. However, Horai et al and Inaba et al[13,14] and Ieiri et al[15] reported that CYP2C19 genotypic differences affected the metabolism and kinetics process of RPZ, and influenced gastric pH values and gastrin level in plasma. In the present study, we found that the AUC for RPZ differed markedly only between homEM and PM, and the intragastric pH, the best and most direct pharmacological index when using PPI, was not significantly different among the different genotypes after a single or repeated doses of RPZ. As for the discrepancy between the kinetics and dynamics of RPZ, first we may hypothesize that the acid-inhibitory effect of RPZ is powerful and rapid, and that the serum levels of 20 mg RPZ are sufficient for acid-inhibitory efficacy in Chinese subjects, even in homEM subjects. Second, no direct and simple relationship between the serum concentration–time profile of the drug and the pharmacodynamic response has been reported because of the irreversible blockade of the therapeutic target by PPI. However, Hussein et al[16] have shown a clear relationship using the maximum effect (Emax) model. According to the model, our study suggests a lower half-maximal effective AUC value (EAUC50) for RPZ than 2 mg·L-1·h. As shown in Table 3, the mean AUC values of RPZ corresponding to the maximum acid-inhibitory effect of RPZ in this study may be greater than this threshold. Therefore, there were no significant differences for intragastric pH among the three geno-types.
In conclusion, our study focused on investigating the pharmacodynamic and pharmacokinetic effect of RPZ with reference to different CYP2C19 genotypes. The acid-inhibitory effects of RPZ were independent on their pharmacokinetic characteristics as well as an individual’s CYP2C19 genotype status. Therefore, RPZ may be a more effective PPI for treating acid-related disease in relation to CYP2C19 genotype status.
References
- Andersson T, Regardh CG, Dahl-Puustinen ML, Bertilsson L. Slow omeprazole metabolizers are also poor S-mephenytoin hydroxylators. Ther Drug Monit 1990;12:415-6.
- Chiba K, Kobayashi K, Manabe K, Tani M, Kamataki T, Ishizaki T. Oxidative metabolism of omeprazole in human liver microsomes: cosegregation with S-mephenytoin 4’-hydroxylation. J Pharmacol Exp Ther 1993;266:52-9.
- Adachi K, Katsube T, Kawamura A, Takashima T, Yuki M, Amano K, et al. CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther 2000;14:1259-66.
- Sakai T, Aoyama N, Kita T, Sakaeda T, Nishiguchi K, Nishitora Y, et al. CYP2C19 genotype and pharmacokinetics of three proton pump inhibitors in healthy subjects. Pharm Res 2001;18:721-7.
- Hokari K, Sugiyama T, Kato M, Saito M, Miyagishima T, Kudo M, et al. Efficacy of triple therapy with rabeprazole for Helicobacter pylori infection and CYP2C19 genetic polymorphism. Aliment Pharmacol Ther 2001;15:1479-84.
- Miyoshi M, Mizuno M, Ishiki K, Nagahara Y, Maga T, Torigoe T, et al. A randomized open trial for comparison of proton pump inhibitors, omeprazole versus rabeprazole, in dual therapy for Helicobacter pylori infection in relation to CYP2C19 genetic polymorphism. J Gastroenterol Hepatol 2001;16:723-8.
- Shu Y, Zhou HH. Individual and ethnic differences in CYP2C19 activity in Chinese populations. Acta Pharmacol Sin 2000;21:193-9.
- de Morais SM, Goldstein JA, Xie HG, Huang SL, Lu YQ, Xia H, et al. Genetic analysis of the S-mephenytoin polymorphism in a Chinese population. Clin Pharmacol Ther 1995;58:404-11.
- Yasuda S, Ohnishi A, Ogawa T, Tomono Y, Hasegawa J, Nakai H, et al. Pharmacokinetic properties of E3810, a new proton pump inhibitor, in healthy male volunteers. Int J Clin Pharmacol Ther 1994;32:466-73.
- Ishizaki T, Chiba K, Manabe K, Koyama E, Hayashi M, Yasuda S, et al. Comparison of interaction potential of a new proton pump inhibitor, E3810, versus omeprazole with diazepam in extensive and poor metabolizers of S-mephenytoin 4’-hydroxylation. Clin Pharmacol Ther 1995;58:155-64.
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors–emphasis on rabeprazole. Aliment Pharmacol Ther 1999;13 Suppl 3:27-36.
- Williams MP, Sercombe J, Hamilton MI, Pounder RE. A placebo-controlled trial to assess the effects of 8 days of dosing with rabeprazole versus omeprazole on 24-h intragastric acidity and plasma gastrin concentrations in young healthy male subjects. Aliment Pharmacol Ther 1998;12:1079-89.
- Horai Y, Kimura M, Furuie H, Matsuguma K, Irie S, Koga Y, et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther 2001;15:793-803.
- Inaba T, Mizuno M, Kawai K, Yokota K, Oguma K, Miyoshi M, et al. Randomized open trial for comparison of proton pump inhibitors in triple therapy for Helicobacter pylori infection in relation to CYP2C19 genotype. J Gastroenterol Hepatol 2002;17:748-53.
- Ieiri I, Kishimoto Y, Okochi H, Momiyama K, Morita T, Kitano M, et al. Comparison of the kinetic disposition of and serum gastrin change by lansoprazole versus rabeprazole during an 8-day dosing scheme in relation to CYP2C19 polymorphism. Eur J Clin Pharmacol 2001;57:485-92.
- Hussein Z, Granneman GR, Mukherjee D, Samara E, Hogan DL, Koss MA, et al. Age-related differences in the pharmacokinetics and pharmacodynamics of lansoprazole. Br J Clin Pharmacol 1993;36:391-8.
