Gene expression profile induced by oral administration of baicalin and gardenin after focal brain ischemia in rats1
Introduction
Stroke, mostly caused by cerebral ischemia, is a multifactorial disease involving the activation of myriads of death inducers, which leads to injury of neurons by eliciting cascades of signal transduction pathways[1]. The Qing Kai Ling injection (QKLI) is a modified preparation of the “An Gong Niu Huang” pill, a famous traditional Chinese medicament. In recent studies, QKLI showed strong therapeutic effects on stroke in clinical usage. However, this preparation is a compound comprising of many components. Its therapeutic mechanism is very complex, with regard to both individual and integrative effects. Baicalin and gardenin are two effective compounds of QKLI. Baicalin, known as an antioxidant flavonoid in vitro, functions as a biological response modifier[2,3], and has been investigated for its neuroprotective effects against glutamate/N-methyl-D-aspartate (Glu/NMDA) stimulation and glucose deprivation in primary cultured rat brain neurons. Baicalin was found to significantly reduce Glu/NMDA-increased lactate dehydrogenase (LDH) release[4]. Baicalin is also a potent inhibitor of endothelial cell prolifera-tion, migration, and differentiation[5]. Gardenin can significantly decrease the content of transcription factor monocyte chemoattractant protein-1 (MCP-1) in rat brains suffering focal ischemia[6]. cDNA microarray possesses the ability to analyze the expression changes of hundreds, thousands, or even tens of thousands of genes simultaneously. Several waves of gene expression have been observed after focal cerebral ischemia[7]; however, a comparative study examining the pharmacological mechanisms of different drugs has not been reported in the literature. Results from the present study suggest that baicalin and gardenin play a pharmacological role in focal cerebral ischemia by regulating gene expression.
Materials and methods
Cerebral ischemia Forty-four Sprague-Dawley rats (250–280 g, supplied by Beijing Weitong-Lihua Experimental Animal Center, Beijing, China) were randomly divided into four groups: model, baicalin-treatment, gardenin-treatment, and sham-operated group, respectively. Under isofurane anesthesia, rats were subjected to occlusion of the left middle cerebral artery (MCAO) using an intraluminal filament as described by Haruo et al[8]. After 24 h of focal cerebral ischemia, the rats were deeply re-anesthetized using halothane and the left half of the brain was removed for total RNA isolation. In the sham-operated rats, all procedures except for MCAO were carried out. Baicalin or gardenin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) was given orally (40 mg/kg) 2 h after MCAO.
Measurement of cerebral infarction areas Eight rats from each group were used to calculate the infarction ratio. In brief, the cerebrum was removed and cut into five slices on the coronal section at the location from which the distance to the prefrontal is 1, 3, 5, and 7 mm, respectively. The slices were stained in 4% 2,3,5-triphenyltetrazolium chloride (TTC) solution for 10 min[9]. Images of the slices were captured using a digital camera (Color CCD camera TP-6001A, Topica Inc, Japan). We calculated the areas of the infarction region using a Pathology Image Analysis System (Topica Inc, Japan). The ratio of the infarction area to the total slice area was calculated.
Total RNA isolation The left half of the brain from the remaining three rats in each group was carefully dissected out from the re-anesthetized rats under RNAase-free conditions. The total RNA in each sample was extracted with Trizol reagent (Invitrogen, San Diego, CA, USA) using the procedure outlined in the manufacturer’s protocol.
Microarrays Hybridization was carried out on the rat “Biostar40S” gene microarray (BioStar Genechip Incorporated, Shanghai, China), which contained 4096 elements. The array provided a broad overview of the rat genome, which was added to the high-quality array cDNA libraries, and the sequence information, maps, and expression data were placed into the public domain (NCBI, Nucleotide). All clones used for production of the microarrays were sequence verified. Three pieces of total RNA from the same group were pooled following transcription into cDNA and were labeled with Cy5 and Cy3, respectively, and hybridized on the array. Image files were processed using the Axon GenePix 4000B scanner(Axon, USA) and datasets were prepared according to the routine procedures using Genepix 4.0 software (Axon, USA).
Data analysis Data sheets derived from the results of the Genepix 4.0 analysis were further evaluated using Excel software (Microsoft, USA). The microarrays were hybridized in triplicate and each measurement containing the extracted total RNA from each group was used to analyze each gene, which had three data points of relative changes. Thus, a balance coefficient was calculated to correct variations resulting from unequal amounts of fluorescent dye fade; the average signal from all elements in the Cy3 channel was divided by the average signal from all elements in the Cy5 channel, which resulted in the balance coefficient. The Cy5 signal for each element was then multiplied by the balance coefficient, prior to calculating the expression ratio (Cy3/Cy5). A fold value of =2.0 or = -2.0 indicated that differences in the Cy3:Cy5 ratio were detected at a 99% confidence level (information provided by Incyte, USA). All probes fulfilling the criterion (=2.0 or = -2.0) were compiled[10–13].
Semiquantitative RT-PCR The procedures for isolating total RNA using Trizol reagent (Invitrogen, Life Technology, Incorporated, New York, NY, USA) and synthesizing first strand cDNA were exactly as previously described[14]. The optimal polymerase chain reaction (PCR) amplification conditions and cycle number were determined experimentally to ensure specific signal generation. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was considered to be stably expressed after focal cerebral ischemia acted as an inter-reference gene. All amplifications were carried out on a Gradient PCR System (Biometra, USA). cDNA synthesis was carried out using 200 ng of mRNA with 5×106 U/L reverse transcriptase (TaKaRa, Japan) in a total 100 μL reaction mixture and incubated for 60 min at 48 °C. The primer pairs were for ribosomal protein L19 (Rpl19): AAC AGA TCA GGA AGC TGA TCA AGA; AGT CTT GAT GAT CTC CTC CTT CTT (NM 031103); casein kinase II beta subunit (Csnk2b): CGG ACA TAA AGA TGA GTA GCT CTG A; GTG GTG CCT AGA GGA CTT GGG TGT G (NM 031021); GAPDH: ACC ACA GTC CAT GCC ATC AC; TCC ACC ACC CTG TTG CTG TA (NM 008084.1). After heating to 95 °C for 5 min, each RT reaction mixture was used for PCR amplification. The PCR mixture started with in an initial step of 5 min at 95 °C followed by 35 cycles of 30 s at 95 °C and an annealing temperature of 1 min at 64.5 °C for Csnk2b mRNA, at 57.4 °C for Rpl19 mRNA and GAPDH, followed by 72 °C for 10 min. Products of 5 μL from each PCR mixture were dyed with 1 μL SYBR Green I Nucleic Acid Gel stain (Cambrex Bio Science Rockland Incorporated, USA) and loaded on 2.5% agarose gels, which were evaluated according to the intensity differences of image electrophoresis using Gel Analyzing Imager (FuRi, Shanghai, China).
Results
TTC staining Both baicalin and gardenin reduced the infarction areas in cerebral ischemia rats by 6%–7% compared with the model group. The ratio of differences was tested using the Student t-test (Figure 1, Table 1).
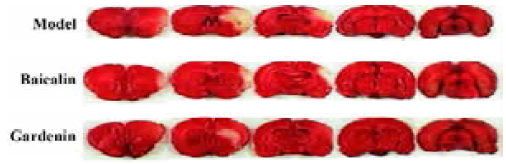
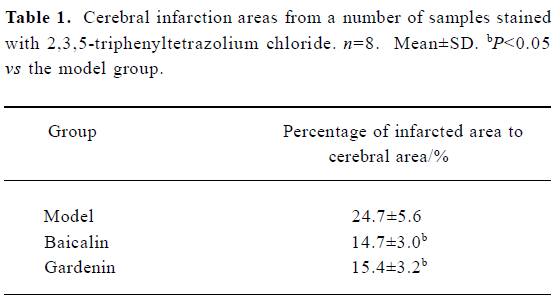
Full table
Changes in gene expression after MCAO occlusion Genes were classified into 12 categories by the International General Principle (www.geneontology.org). One hundred and ninety-nine genes were significantly upregulated which are involved in metabolism, signal transduction, cell organization, response to stress, cell adhesion, transport, apoptosis, and a variety of other processes. Another 12 genes showed downregulated expression under the same conditions (Table 2).
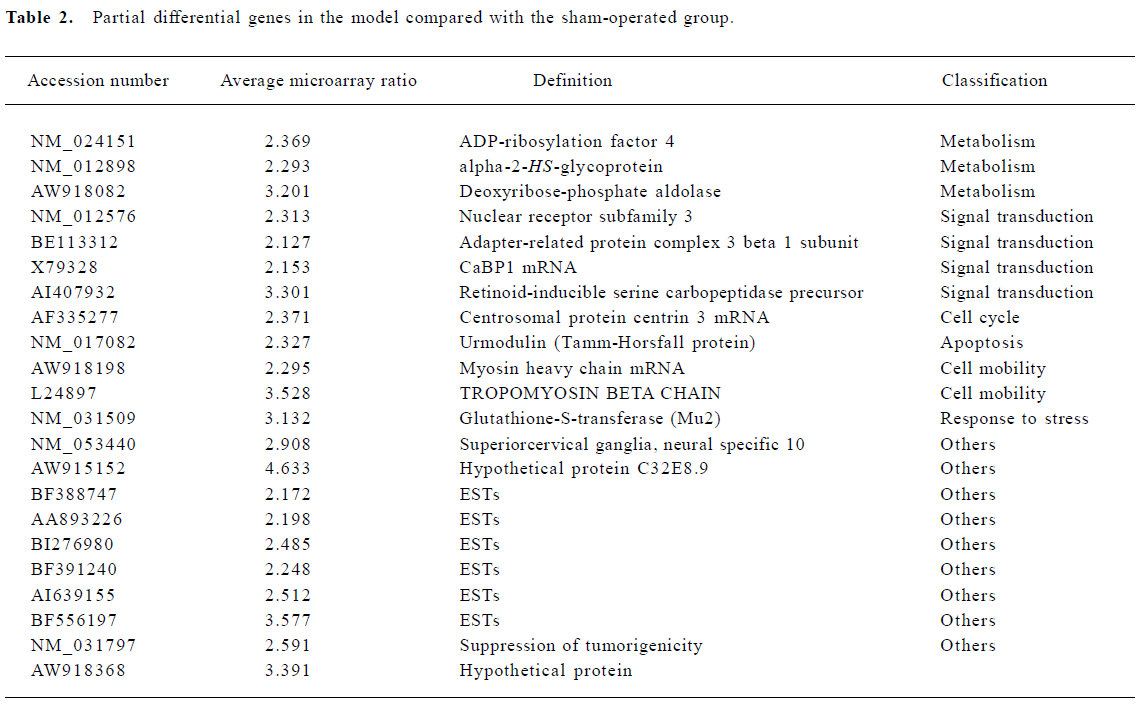
Full table
Metabolism The largest increases in gene expression were shown by genes involved in protein synthesis: the ribosomal proteins L10, S5, S3a, S24, S6, S11, and L19, glycoprotein 38, eukaryotic translation elongation factor 1 alpha and actin-related protein complex 1b. The expression of genes related to cell metabolism changed with increased expression of coenzyme A dehydrogenase, procollagen-lysine, lactate dehydrogenase and ubiquinone oxidoreductase subunit B13.
Cell organization and adhesion Cell organization and adhesion genes also showed prominent changes in expression. These included Fibronectin 1, Integrin, H2A histone family member and apolipoprotein M.
Signal transduction Increases in expression were shown by genes involved in cellular signal transduction: CDK5, pyruvate kinase 3, phosphatidylinositol 4-kinase, and casein kinase II. In addition, the G protein pathway suppressor showed decreased expression over this interval.
Response to stress Several “stress” genes involved in cellular responses to inflammation and injury were induced, including Cd63 antigen, MRC OX-45 surface antigen and immunoglobulin superfamily member. Another induced gene, glutathione peroxidase 1, was also upregulated after focal cerebral ischemia.
Apoptotic genes The expression of several apoptotic effector genes was altered. These included programmed-cell-death-8, Urmodulin, and tumor protein translationally controlled genes.
Effect of baicalin on gene expression Increases in gene expression were observed in 89 genes. These included genes involved in metabolism, signal transduction, cell organization, responses to stress, and transcription regulators. In addition to the induced genes, 88 genes simultaneously showed decreased expression (Table 3).
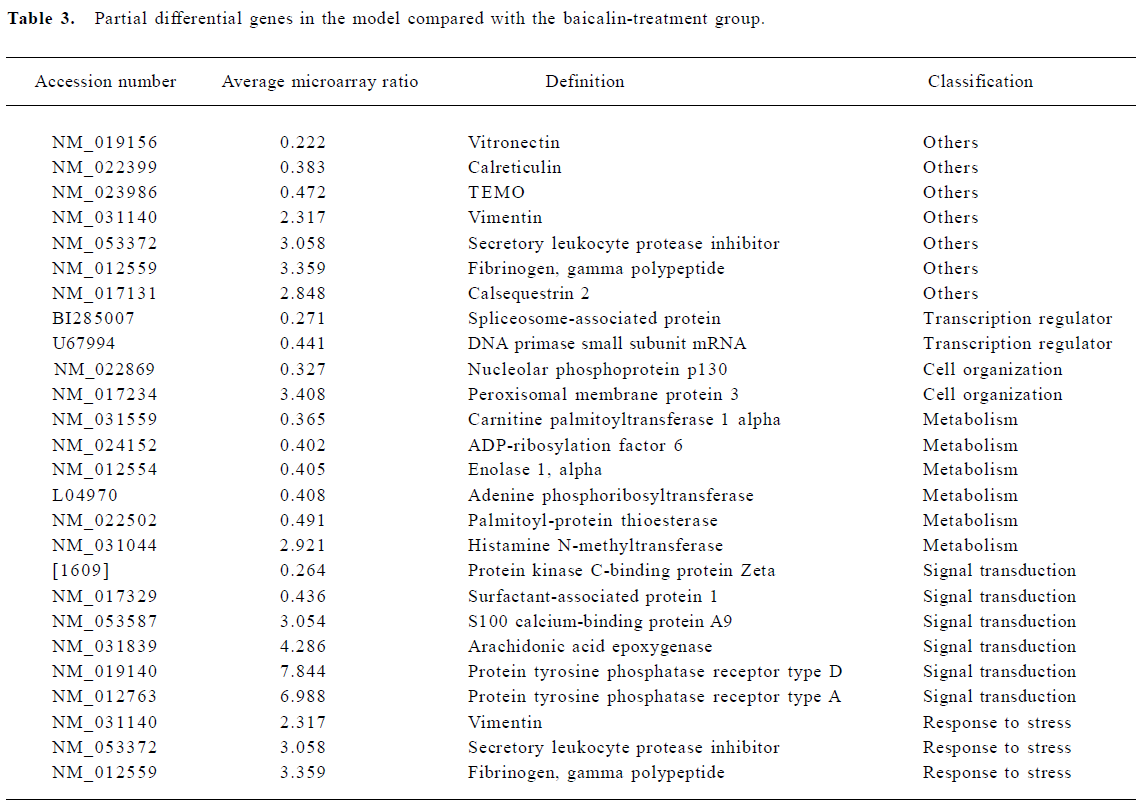
Full table
Metabolism Metabolism-related genes showed prominent changes in expression. These included ADP-ribosyla-tion factor, enolase, adenine phosphoribosyltransferase, and palmitoyl-protein thioesterase. In addition, histamine N-methyltransferase simultaneously showed decreased expression.
Signal transduction The largest increases in expression were shown by genes involved in protein tyrosine phosphatase receptor type D and receptor type A. S100 calcium-binding protein A9 also showed prominent changes. In addition, the expression of protein kinase C-binding protein Zeta and surfactant-associated protein decreased. Protein kinase is controlled by specific binding proteins, which are believed to sequester each type of kinase to the region of a neuron, such as the postsynaptic specialization or cell nucleus, that requires its function[15]. The protein kinase C-binding protein Zeta is one of these proteins. Arachidonic acid epoxygenase, an adapter protein of the prostaglandin and leukotriene family of intracellular messengers, also appears to play an important role in the regulation of signal transduction in the brain and elsewhere[14].
Cell organization The expression of nucleolar phosphoprotein p130, an adapter protein that participates in nucleolar disassembly and cell cycle, decreased after MCAO. In addition, peroxisomal membrane protein showed increased expression over this interval.
Transcription regulator Two genes related to the transcription regulator were altered. These were spliceosome-associated protein and DNA primase.
Effect of gardenin on gene expression Increases in gene expression were observed for 68 genes. These included genes involved in metabolism, signal transduction, cell organization, response to stress, cell cycle, and cell mobility. In addition, two genes showed decreased expression over this interval (Table 4).
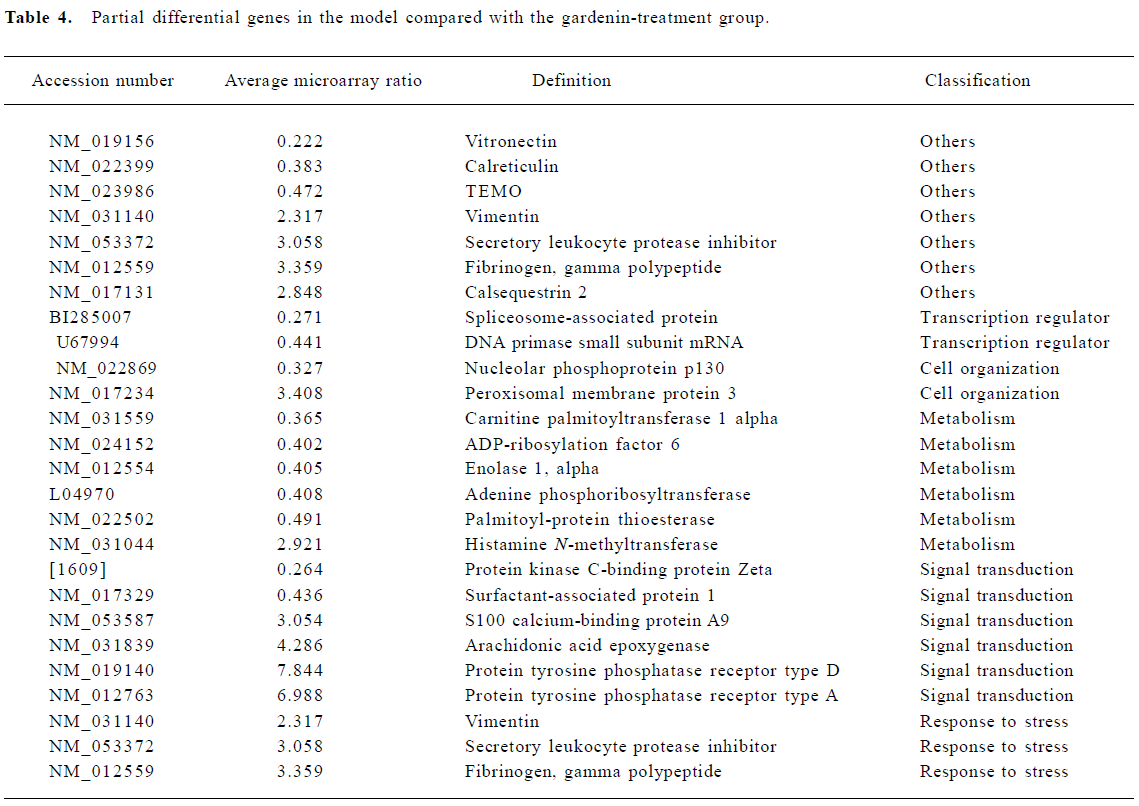
Full table
Metabolism The expression of several genes involved in protein synthesis and cell metabolism was altered. These included ADP-ribosylation factor, HS-glycoprotein and deoxyribose-phosphate aldolase.
Signal transduction Changes in the expression of genes indirectly related to signal transduction were observed, and consisted of increased expression in nuclear receptor, adapter-related protein complex and retinoid-inducible serine carbopeptidase precursor.
Response to stress Several “stress” genes involved in cellular responses to oxidation were induced. The most striking was the increased expression of glutathione-S-trans-ferase.
Cell mobility Myosin heavy chain and tropomyosin beta chain genes that participate in cell mobility and cell cycle were also induced.
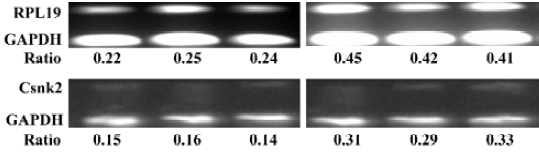
Semiquantitative RT-PCR The outcome of this study showed that the expression of ribosomal protein RpL19 mRNA and Csnk2 mRNA were consistently upregulated in the individual samples from MCAO rats relative to sham-operated rats. Expression increased by 1.9- and 2.1-fold, respectively, and showed the same trend as the results of the microarray. The outcome of RT-PCR indicated that broad, array-based gene expression measurements were reliable for determining gene expression patterns in the brain (Figure 2).
Discussion
Messenger RNA is only an intermediate on the way to the production of the eventual protein products. In the present study we explored the potential role of cDNA microarrays for gene expression analysis after focal brain ischemia and examined the differences in gene expression after the action of different compounds of QKLI. An ischemia period of 24 h was used because this is the maximum period of cerebral ischemia that is compatible with neuroprotection[16–18]. To select results from microarray experiments with reliably altered ratios, we filtered the results using two criteria[10–13]: minimal fold-change values, and ratios reproducibly different from unity. The hybridization ratio had to be at least two-fold higher or lower than the control groups. Of these filters, the two-fold ratio filter was by far the most restrictive and there was no uniform standard across the different platforms of the arrays. We have listed the information of differential gene expression according to the functional classification of genes. In brief, baicalin and gardenin appear to have a profound influence on the model by regulating the expression of different genes or by acting on different metabolic pathways. The genes related to cell metabolism presented striking changes, and showed increased expression in both model and baicalin-treatment groups in contrast to the gardenin-treatment group. Baicalin appears to have a more prominent action on signal transduction in cells than gardenin, including activation of ion channels, regulation of kinase and phosphorylation of receptor proteins. In contrast, the effects of gardenin in stress responses and anti-oxidation appear to be more significant than those of baicalin.
Changes in gene expression after MCAO Prominent changes were recorded in the ribosomal proteins S6, L6, S3a, S24, L5, L10, L19, and S11 on the microarray[19]. In general, this finding supports previous studies[20], perhaps reflecting recovery from an early postischemic transcriptional defect. Integrin is a transmembrane protein, and the adhesion effects between leucocytes and cerebral micrangium cells mediated by integrin participated in the injury and destruction of inflammation factors to tissues. The increased expression of integrin after MCAO is, in general, in line with a preceding report[21]. The increased expression of protein kinase (PK) is mostly caused by the large amount of PK released as a result of metabolic dysfunction, necrosis, ischemia and hypoxia of cells, which occurs in acute cerebrovascular diseases, and this finding was in accordance with the clinical diagnosis. The increased expression of genes in the G protein pathway suppressor 1 may have contributed to the adaptive modulation of the body. In general, G protein plays a role in signal amplification and in switching the molecule on and off in the course of signal transduction. G protein pathway suppressor was upregulated after focal cerebral ischemia, which might occur in compensation for stress and signal transduction regulation in the body[22,23]. Casein kinase II is a necessary substance for cell survival, and increased expression of casein kinease II is correlated with hyperplasia and the proliferation of cells after cerebral ischemia. Cyclin-dependent kinase 5 (CDK5) mRNA was found to be downregulated after focal brain ischemia, whereas variable changes were noted at the protein level in focal ischemia[24]. Downregulation of phosphatases was noted, which would alter the balance of protein phosphorylation in several cellular signaling pathways; much more information is needed before any suggestions regarding functional effects can be made. As the oxidation of glutathione peroxidase might offer a new modulating mechanism of cellular signal transduction[25], it was suggested that its increased expression could provide a sign in response to stress in the body.
Effect of baicalin on gene expression Programmed-cell-death-8 was upregulated after MCAO with baicalin treatment. Furthermore, novel mediators of cerebral ischemia inducing neuronal death have also been found using cDNA microarrays. All these studies reinforce the idea that common cell death pathways are activated in response to neurondeath inducers, which promise to serve as potential therapeutic targets for modulation to achieve neuroprotection[26]. The expression of the protein kinase C-binding protein Zeta was downregulated in rats treated with baicalin, which resulted in a decrease in the activity of protein kinase C (PKC). Aronowski et al[27] observed that the activity of PKC in the brain cortex and hippocampus decreased significantly after ischemia and this decrease could be alleviated by the pre-treatment of the NMDA receptor antagonist. Further studies need to examine whether baicalin acts as an antagonist to the NMDA receptor. After all, the relationship between the changing activity of PKC and neuron injury has not been elucidated completely in many key pathophysiological procedures of ischemia, such as increment of fermentation, acidosis, and deficiency of ATP production. The prominent differential genes involved in protein tyrosine phosphatase receptor type D and A are central to the course of signal transduction, which has been implicated both in the regulation of cell growth and the rearrangement of actin that is mediated by several receptor tyrosine kinases. Differential gene expression showed that baicalin played an important role in cell signal transduction and protein phosphorylation after MCAO, and might act as a neuroprotectant.
Effect of gardenin on gene expression The differential genes in the gardenin-treatment group showed extreme variation compared with the baicalin-treatment group. Several genes encoding anti-oxidation were upregulated. For example, an increase in Mu2 suggested that Mu2 played an activation role in anti-oxidation responses, and was regarded as one of the markers of internal anti-injury because of its anti-oxidative and antidotal effects[25]. According to the actions of Mu2, the anti-oxidative and antidotal effects of gardenin might be one of the mechanisms of cerebral protection after MCAO.
The most interesting phenomenon observed was that there was no overlap in the genes showing differential expression in the three groups despite the similar trends in expression. The results suggest that there are considerable differences in the pharmacological effects of baicalin and gardenin at the molecular level after MCAO. Compared with the role of gardenin and bacalin, which are components of QKLI, it appears that QKLI plays an integral role and has important therapeutic effects requiring further investigation.
In conclusion, the cDNA microarray study not only confirmed that changes in many genes contributed to cerebral ischemia, but also suggested several potential targets for further investigation. Of course, global expression of genes measured at the levels of mRNA transcription obtained using microarray analysis should be viewed from two different standpoints. Looking from mRNA towards protein, one would ultimately like to test the increased production of the proteins encoded by the upregulated mRNA or demonstrate a loss of protein encoded by downregulated mRNA. Thus, it may be rewarding to reconstruct the networks of regulation in response to ischemia and, by inference, to hypoxia, using bioinformatic strategies[28]. Understanding such regulatory networks and the therapeutic mechanisms of QKLI for ischemia–hypoxia responsive gene expression in neurons may extend the relevance of these studies.
References
- Hou ST, MacManus JP. Molecular mechanisms of cerebral ischemia-induced neuronal death. Int Rev Cytol 2002;221:93-148.
- Chen X, Nishida H, Konishi T. Baicalin promoted the repair of DNA single strand breakage caused by H2O2 in cultured NIH3T3 fibroblasts. Biol Pharm Bull 2003;26:282-4.
- Huang Y, Wong CM, Lau CW, Yao X, Tsang SY, Su YL, et al. Inhibition of nitric oxide/cyclic GMP-mediated relaxation by purified flavonoids, baicalin and baicalein in rat aortic rings. Biochem Pharmacol 2004;67:787-94.
- Lee HH, Yang LL, Wang CC, Hu SY, Chang SF, Lee YH. Differential effects of natural polyphenols on neuronal survival in primary cultured central neurons against glutamate- and glucose deprivation-induced neuronal death. Brain Res 2003;986:103-15.
- Liu JJ, Huang TS, Cheng WF, Lu FJ. Baicalein and baicalin are potent inhibitors of angiogenesis: inhibition of endothelial cell proliferation, migration and differentiation. Int J Cancer 2003;106:559-65.
- Zhou JW. Gardenin and bacalin show the effect on MCP-1 of rat brain suffered focal ischemia. Chin Arch Tradit Chin Med 2004;22:1016-7.
- Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab 2000;20:1011-32.
- Haruo N, Agasawa K, Yuya K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke 1989;20:1037-42.
- Bederson JB. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986;17:1304-8.
- Jiang CH, Tsien JZ, Schultz PG, Hu Y. The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci USA 2001;98:1930-4.
- Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, et al. Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol 2001;50:93-103.
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet 2000;25:294-7.
- Sandberg R, Yasuda R, Pankratz DP, Barlow C. Regional and strain-specific gene expression mapping in the adult mouse brain. Proc Natl Acad Sci USA 2000;97:11038-43.
- Hou ST, Callaghan D, Fournier MC, Hill I, Kang L, Massie B, et al. The transcription factor E2F1 modulates apoptosis of neurons. J Neurochem 2000;75:91-100.
- Jaken S. An overlay assay for detecting protein kinase C-binding proteins and substrates. Methods Mol Biol 2003;233:359-68.
- Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke 2001;32:553-60.
- Huh PW, Belayev L, Zhao W, Koch S, Busto R, Ginsberg MD. Comparative neuroprotective efficacy of prolonged moderate intraischemic and postischemic hypothermia in focal cerebral ischemia. J Neurosurg 2000;92:91-9.
- Belayev L, Zhao W, Busto R, Ginsberg MD. Transient middle cerebral artery occlusion by intraluminal suture: I. Three-dimensional autoradiographic image-analysis of local cerebral glucose metabolism-blood flow interrelationships during ischemia and early recirculation. J Cereb Blood Flow Metab 1997;17:1266-80.
- Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene 2001;265:11-23.
- Schmidt-Kastner R, Zhang B, Belayev L, Khoutorova L, Amin R, Busto R, et al. DNA microarray analysis of cortical gene expression during early recirculation after focal brain ischemia in rat. Brain Res Mol Brain Res 2002;108:81-93.
- Zhang ZG, Chopp M, Tang WX, Jiang N, Zhang RL. Postischemic treatment (2-4) with anti-CD11b and anti-CD18 monoclonal antibodies is neuroprotective after transient (2 h) focal cerebral ischemia in the rat. Brain Res 1995;698:79-85.
- Bogousslavsky J, Paciaroni M, Gallai V. Glycoprotein (GP) IIb/IIIa inhibitors for acute stroke treatment. Clin Exp Hypertens 2002;24:603-10.
- Wannier-Morino P, Rager G, Sonderegger P, Grabs D. Expression of neuroserpin in the visual cortex of the mouse during the developmental critical period. Eur J Neurosci 2003;17:1853-60.
- Hayashi T, Warita H, Abe K, Itoyama Y. Expression of cyclin dependent kinase 5 and its activator p35 in rat brain after middle cerebral artery occlusion. Neurosci Lett 1999;265:37-40.
- Paolicchi A, Dominici S, Pieri L, Maellaro E, Pompella A. Glutathione catabolism as a signaling mechanism. Biochem Pharma-col 2002;64:1027-35.
- Read SJ, Parsons AA, Harrison DC, Philpott K, Kabnick K, O’Brien S, et al. Stroke genomics: approaches to identify, validate, and understand ischemic stroke gene expression. J Cereb Blood Flow Metab 2001;21:755-78.
- Aronowski J, Waxham MN, Grotta JC. Neuronal protection and preservation of calcium/calmodulin-dependent PKII and PKC activity by dextrorphan treatment in lobal ischemia. J Cereb Blood Flow Metab 1993;13:550-7.
- Thieffry D. From global expression data to gene networks. Bioessays 1999;21:895-9.
